Commentary: Is ADEM a subgroup of MS?
Shinji Ohara
Abstract
Acute disseminated encephalomyelitis (ADEM) and Multiple sclerosis (MS) are both immunologically mediated inflammatory demyelinating disease of the CNS. ADEM and MS have long been considered as a separate disease entities, but clinical differentiation of ADEM from the first attack of MS is often difficult because of overlapping clinical features. Pathologically, perivenous demyelination and discrete confluent demyelination (plaque) have been generally regarded as the hallmark of ADEM and MS, respectively. It is also known that in contrast to MS, which shows quite diverse heterogeneous pathologic patterns, ADEM shows generally homogenous pathological features of inflammatory demyelination. However, hybrid cases showing pathological features of both ADEM and MS do exist, suggesting that ADEM may share some common underlying pathologic mechanisms with certain stages or subgroups of MS.
Text
Acute disseminated encephalomyelitis (ADEM) and multiple sclerosis (MS) have been considered as clinically and pathologically distinct phenotype of inflammatory demyelinating disease of CNS1,2. ADEM, typically but not always anteceded by infectious illness or vaccination, presents with a monophasic course with relatively favorable prognosis, while MS typically exhibits a relapsing and remitting course with accumulating neurological deficits with each exacerbation. However, despite several clinical and/or radiological criteria proposed to differentiate ADEM from MS, none has been proven to unequivocally separate them. With many similarities in clinical presentation, MRI findings and putative pathogenesis, Hartung and Grossmann speculated that ADEM may not be a distinctive disease but a part of the MS spectrum3. On the other hand, pathological differentiation between ADEM and MS has been regarded as most reliable. ADEM is characterized by perivenous demyelination and MS by confluent demyelination, with these two patterns seldom coexisting in a single patient4.
We have recently experienced a patient clinically diagnosed as ADEM whose brain biopsy revealed pathologically features indistinguishable from active lesions of MS in addition to the characteristic foci of perivenular inflammation and demyelination of ADEM5. This mini-review is based on this report.
Case presentation
Detailed clinical information of the patient has been reported previously5. Briefly, a 51-year female presented with progressive aphasia and right-sided hemiparesis after a month history of new onset increasing headache. There was no apparent antecedent flu-like illness or history of vaccination. Cerebrospinal fluid (CSF) examination revealed slight pleocytosis and increase in protein and myelin basic protein (MBP). There were no oligoclonal bands. Brain MRI revealed multifocal subcortical lesions mainly involving subcortical white matter of the left temporal and parietal lobes without enhancement.
Because of the rapid progression of her symptoms with the new onset of focal seizures, a brain biopsy was performed. She was then started on intravenous methyl prednisolone followed by oral prednisolone taper. The patient responded remarkably both clinically and radiologically, and was discharged two month later. She was able to resume her former daily social activities. At the time of this writing, 9 years later, there has been no clinical recurrence.
The brain biopsy revealed both patterns of perivenular/perivascular demyelination and a confluent demyelination in the cerebral cortices and in subcortical white matter. They were associated with perivascular cuffing of B and T lymphocytes. The leptomeninges also revealed an inflammatory cell infiltrates, composed of B and T cells, occasionally infiltrating into the Virchow-Robin spaces. Immunohistochemistry with MBP or CNPase using thicker sections, revealed areas of subpial demyelination5.
The representative perivenous demyelination of this case is shown in the Figure (A-H). They were immunostained on serial sections for various cell markers (CD4; helper T cell marker, CD8; cytotoxic T cell marker, CD20; a B cell marker, HLA-DR; microglial marker). The perivascular inflammatory infiltrates were immunoreactive for HLA-DR, CD20, CD4 and CD8 (E-H). In the area of perivenous demyelination, there was a sheet-like proliferation of HLA-DR positive microglial cells. Activated microglia as manifested by rich cytoplasm and ramified cell processes could also seen around the demyelinating lesion (E). There was a clear tendency of CD20 positive B cells to be localized in the perivascular space limited by glia limitans (B, F). On the other hand, CD4 and CD8 positive T cells were found scattered in the perivenular demyelination and also found infiltrating in the surrounding brain parenchyma (G, H).
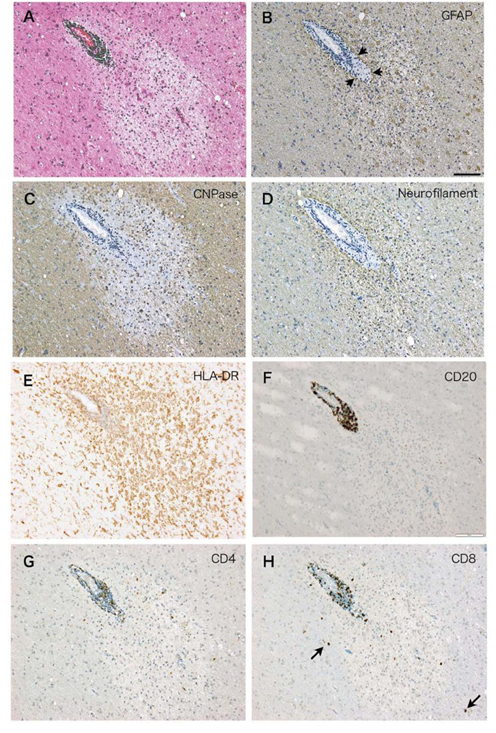
Figure (A-H): Serial sections of the perivenular demyelination characteristic for ADEM in the case4 stained with H.E.(A), and immunostained with antibody to GFAP (B), CNPase (C), neurofilament (D), HLA-DR (E), CD20 (F), CD4 (G), CD8 (H), respectively. In the area of perivenular demyelination, there was an increase in the number of HLA-DR positive microglia (E) and reactive astrocytes (B). Arrowheads in (B) indicate glia limitans defining perivascular infiltrates. CD20+ B cells (F) tend to be restricted in the perivascular space, while CD4+ T cells (G) and CD8+ T cells (H) are more scattered in the area of perivascular demyelination. Arrows in (H) indicate CD8 positive cells apparently outside of the demyelinating lesion. Bar= 50μm.
On the coexistence of ADEM and MS pathology
It remains unsettled is if ADEM is immunopathologically distinct from MS or may share common underlying pathologic mechanisms with MS. Similar controversy also exists concerning distinction of the multiphasic form of ADEM (MDEM) from relapsing MS6. This seems partly because, in contrast to those of MS, few cases of ADEM with immunohistochemical analysis have been available so far, including MEDM variant. A variability in the appearance and nature of T and B lymphocytes has been reported7. In one fulminant case of ADEM, stereotaxic biopsy of the frontal white matter showed perivenous inflammation and demyelination with presence of CD3 positive, CD8 positive, but not CD20 positive B cells8.
In contrast to ADEM, which shows generally homogenous pathological features with known similarities to experimental autoimmune encephalitis, MS is known to be pathologically diverse. According to Lucchinatti, four patterns could be discerned in MS.: Pattern I lesions show inflammatory lesions made up of T cells and macrophages alone: Pattern II lesions contain immunoglobulin and complement: Pattern III is a distal dying back oligodendrogliopathy: and Pattern IV is characterized by primary oligodendroglial degeneration9.
In the case we have reported, both the perivenous demyelination and the confluent demyelination, pathognomic feature for ADEM and MS respectively, were recognized5. The demyelinating pattern of the case we have reported seems most consistent with Pattern I, in that microglia/macrophage and T cells infiltration was evident in the active demyelinating lesions in the absence of IgG deposition by immunohistochemistry, and that there was no apparent features suggestive of oligodendrocyte degeneration. In addition, meningeal inflammation associated with subpial cortical demyelination was also recognized, which have been reported in early and progressive stage of MS10-13. However, the occurrence of leptomeningeal inflammation and subpial demyelination have also been described in ADEM4,14.
Is ADEM a subgroup of MS?
There have been several recent case reports illustrating difficulties in the clinical differentiation between ADEM and MS, in which pathological studies seem to have played a key role.
Hoche et al. reported a 16 year-old patient clinically diagnosed as ADEM whose brain biopsy showed histological features of active MS (pattern I according to Lucchinetti) without perivenous demyelination15.
Popescu et al. reported on a 33 year old patient who started to complain of headache one week after an upper respiratory tract infection. She was found to have a solitary lesion in the occipital cortex and a biopsy demonstrated cortical demyelination associated perivascular infiltrates consisting of T and B cells. The diagnosis of MS was subsequently confirmed through clinical follow up with the new appearance of white matter lesions on MRI10.
Guenther et al. reported an autopsy case of a 19 year-old clinically diagnosed as ADEM who suddenly died of sepsis. Autopsy revealed multiple foci in a confluent demyelination pattern in the absence of areas of perivenular demyelination. The author interpreted that the case could represent either a first demyelinating event in a case of MS with ADEM-like clinical presentation or a case of ADEM with an unusual MS-like pattern of demyelination16.
Yidiz et al. reported a 35 year-old woman with 14-year history of relapsing-remitting MS who developed a hyperacute form of ADEM (Acute Hemorrhagic Leukoencephalitis AHLE), a diagnosis confirmed by brain biopsy. Interestingly, this patient had started experiencing typical MS relapses after the ALHE episode had resolved. The authors believe that MS and ADEM occurred independently in this patient17.
In comparative study of clinical features between those with and without perivenous demyelination using biopsy/autopsy material, Young et al. showed that found that perivenous demyelination is associated with a meningoencephalitic presentation and a monophasic course. Three patients of perivenous demyelination cohort showed both perivenous and confluent patterns and two of them had relapsed. One patient out of ten with perivenous pattern relapsed. On the other hand, 15 out of 91 patients of the confluent cohort showed monophasic course (mean 7.1 years of follow-up), and was diagnosed as isolated demyelinating syndrome4.
Together with these cases illustrating the clinical overlap of MS and ADEM, and the presence of hybrid cases with overlapping pathological features as we have reported, we believe that the possibility of chance association of MS and ADEM seems very unlikely, if not completely excluded. It seems more likely that the CNS pathology of ADEM may share common immunopathologic features of active MS, in which participation of immune effector cells consisting of T and B cells, and activated microglia are essential features18-21. Conceivably, perivenous demyelination and confluent demyelination may have same pathologic basis and differ only in the stage and the appearance of the lesions. Under certain conditions, small perivenous demyelination may evolve and coalesce into a confluent demyelination.
Conclusion
ADEM and MS have been considered as distinct phenotypes, pathologically characterized by perivenous demyelination vs. confluent demyelination. However, cases of the first clinical episode of CNS demyelination showing both pathological features of ADEM and MS do exist, suggesting that CNS pathology of ADEM may share common pathologic mechanism(s) with certain subgroups of MS.
Legend for illustrations
Serial sections of the perivenular demyelination characteristic for ADEM in the case4 stained with H.E.(A), and immunostained with antibody to GFAP (B), CNPase (C), neurofilament (D), HLA-DR (E), CD20 (F), CD4 (G), CD8 (H), respectively. In the area of perivenular demyelination, there was an increase in the number of HLA-DR positive microglia (E) and reactive astrocytes (B). Arrowheads in (B) indicate glia limitans defining perivascular infiltrates. CD20+ B cells (F) tend to be restricted in the perivascular space, while CD4+ T cells (G) and CD8+ T cells (H) are more scattered in the area of perivascular demyelination. Arrows in (H) indicate CD8 positive cells apparently outside of the demyelinating lesion. Bar= 50μm.
References
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis the plaque and its pathogenesis. New Engl J Med. 2006; 354: 942-955.
- Sejvar JJ. Acute disseminated encephalomyelitis. Curr Infect Dis Rep. 2008; 10: 307-314.
- Hartung HP, Grossman RI. ADEM distinct disease or part of MS spectrum. Neurology 2001; 56: 1257-1260.
- Young NP, Weinshenker BG, Parisi JE, et al. Perivenous demyelination: associated with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain. 2010; 133: 333-348.
- Koshihara H, Oguchi K, Takei Y, et al. Meningeal inflammation and demyelination in a patient clinically diagnoses as acute disseminated encephalomyelitis. J Neurol Sci. 2014; 346: 323-327.
- Koelman DLH, Mateen FJ. Acute disseminated encephalomyelitis: current controversies in diagnosis and outcome. J Neurol. 2015; 262: 2013-2024.
- Kuhlmann T, Lassmann H, Bruck W. Diagnosis of inflammatory demyelination in biopsy specimens; a practical approach. Acta Neuropathol. 2008; 115: 275-287.
- Schirmer L, Seifert CL, Pfeifenbring S, et al. Clinicopathological considerations in acute disseminated encephalomyelitis (ADEM): a fulminant case with favorable outcome. 11 J Neurol. 2012; 259: 753-755.
- Lucchinetti C, Brück W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000 ;47: 707-17.
- Popescu BFGh, Bunyan RF, Parisi JE, et al. A case of multiple sclerosis presenting with inflammatory cortical demyelination. Neurology. 2011; 76: 1705-1710.
- Howell OW, Reeve CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011; 134; 2755-2771.
- Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. New Engl J Med. 2011; 365: 2188-2197.
- Disanto G, Morahan IM, Barnett MH, et al. The evidence for a role of B cells in multiple sclerosis. Neurology. 2012; 78: 823-832.
- Moore CRW, Stadelmann-Nessler C. Acute disseminated encephalomyelitis and related disorders. In Greenfield Neuropathology 9th ed. CRC Press. 2015; pp 1379-1384.
- Hoche F, Pfeifenbring S, Vlaho S, et al. Rare brain biopsy findings in a first ADEM-like event of pediatric MS histopathologic neuroradiologic and clinical features. J Neural Transm. 2011; 118: 1311-1317.
- Guenther AD, Munoz DG. Plaque-like demyelination in acute disseminated encephalomyelitis (ADEM) – an autopsy case report. Clin Neuropathol. 2013; 32: 486-491.
- Yidiz O, Pul R, Raab P, et al. Acute hemorrhagic leukoencephalitis (Weston-Hurst syndrome) in a patient with relapse-remitting multiple sclerosis. J Neuroinflammation. 2015; 12: 175.
- Booss J, Esiri MM, Tourtelotte WW, et al. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic multiple sclerosis. J Neurol Sci. 1983; 62: 219-32.
- Hauser SL, Bhan AK, Gilles F, et al. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986; 19: 578-87.
- Babbe H, Roers A, Waisman A, et al. Clonal expansion of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000; 192: 393-404.
- Grigoriadis N, van Pesch V. A basic overview of multiple sclerosis immunopathology. Eur J Neurol. 2015; 22: 3-13.
