Frequency-Specific Biomarkers in Neurodegenerative Disorders: Implications of Alpha and Beta Oscillations in Motor Behaviour
Rémy Cohan2,8, Karolina A. Bearss1,2,4,6, Joseph F.X. DeSouza1-8
1Centre for Vision Research, York University, Canada
2Department of Psychology, York University, Canada
3Department of Biology, York University, Canada
4Neuroscience Graduate Diploma Program, York University, Canada
5Interdisciplinary Graduate Studies, York University, Canada
6Canadian Action and Perception Network (CAPnet), York University, Canada
7Vision: Science to Applications (VISTA) Program, York University, Canada
8Multisensory Neuroscience Laboratory, York University, Canada
Abstract
Despite advancement in neuroimaging, the link between motor and cognitive processes, and the role of oscillations in motor behaviour remain unclear. Current research in neurodegenerative disorders (e.g., Parkinson’s and Alzheimer’s disease) indicates that changes in oscillatory brain rhythms (OBRs) observed from electroencephalographic (EEG) studies could be utilized to quantify and understand the neural network changes in the presence of pathology. Research suggests, that rhythmicity is a common feature amongst biological entities, and cyclic fluctuations in neurological systems in response to incoming stimuli from the environment, grant a great degree of flexibility to such systems in order to interact with their surroundings at an optimal level. This reciprocity between exogenous stimuli and endogenous mechanisms in the brain creates a two-way pathway that awards a bi-directional relationship between the environment, and the brain. Here, in this mini review we explore the role of OBRs, and review the current literature supporting the putative role of frequency-specific OBRs as potential biomarkers in neurodegenerative disorders, mainly Parkinson’s disease (PD), which in turn, may allow clinicians to identify effective therapies based on these biomarkers, expanding the armamentarium for delaying the rate of disease progression and symptom management.
Introduction
Oscillatory brain rhythms (OBRs) have served as a window into the electrophysiological activities of the central nervous system since the invention of EEG16, 17. Oscillations are not unique to the brain, they are seen in the physical and biological world from the planetary motion to the sleep-wake cycle, and these oscillations like many other perpetual rhythmic physiological markers follow a cyclic pattern which makes them predictable25. Electrophysiologists have long been taking advantage of the predictability of oscillations in humans. Electrocardiogram for example, measures the polarization and depolarization patterns in cardiac muscles allowing clinicians to indirectly determine and predict structural, and electrical abnormalities of the heart, or could be used to measure behavioural changes in response to stress or pleasure through the rhythms of the heart13. Another characteristic of oscillations is their ability to be entrained19. OBRs change in response to the incoming information from the environment. In fact, human sensation and perception function based on this principle, this is why we are good at interacting with our environment and respond effectively to the changes around us16. We see the flickering light coming from a halogen bulb, or movies shot at 24-48 frames per second as smooth and continuous light or image on the screen (sensory entrainment), or we attempt to phase shift our sleep-wake cycle by taking melatonin (chemical entrainment)13,19. These changes have been measured in EEG studies using frequency-specific OBRs. Before delving deeper into understanding the role of oscillations in quantifying behaviour, it is important to note, that the rhythmic electrical potentials recorded from the scalp electrodes are the summation of excitatory and inhibitory current changes called the local field potentials (LFPs) from clusters of millions of cortical neurons16. Although the application of EEG has been proven to be helpful in diagnostics (e.g., most common way to diagnose epilepsy)6 and biotechnology (using EEG signals in brain-computer interface)19, lack of accurate spatial resolution poses a risk in attributing the results to a specific location in comparison with highly localized and accurate direct intra and extracellular LFP recordings of neurons17. Though with the advent of sophisticated preprocessing and signal analysis techniques, when EEG, fMRI and behavioural studies paired together we begin to see a pattern of correlation between cognitive processes, motor behaviour, and EEG recordings. In this mini review we are not suggesting that OBRs captured on the EEG can yield to an accurate conclusion on modulatory mechanisms and underlying structures involved in motor behaviour but as an indirect measure (biomarker) of motor behaviour. Researchers have been able to identify 5 main frequency bands: delta (? 1-4 Hz), theta (? 4-12 Hz), alpha (? 8-12 Hz), beta (? 13-30 Hz), and gamma (? 30-50 Hz)13,44. Using various signal processing techniques, these oscillations are separated from artifacts and signals picked up from the depolarization of muscles cells19, ready to be used for diagnostic and research purposes. Current literature suggests that power changes in alpha and beta frequency bands (as well as gamma frequencies) could be indicative of network-specific deterioration in aging, neurodegenerative diseases, and some neuropsychiatric disorders (e.g., Schizophrenia and major depression disorders)2,6,16,17,45. In task dependent EEG or event-related potential (ERP) studies, these frequencies are often measured based on event-related synchronization (ERS), where the spectral power increases, and event-related desynchronization (ERD) as the spectral power decreases4,44. Alpha frequency band is thought to be involved in top-down attentional control, and the proponents of the gating by inhibition hypothesis believe that alpha is a neural signature for inhibition of attention to incoming stimuli2,6. ERP studies have shown, that increase in alpha power (ERS) is associated with regular neural activity in the absence of heightened levels of arousal and cognitive load, whereas deliberate shifts in attentional resources, and engagement in tasks demanding high cognitive load leads to a decrease in alpha power (ERD)30, 44. Beta frequency band follows similar patterns, and current literature proposes that beta ERS and ERD are correlated with motor mastery, or the effortless execution of a motor task23,40,44. Experimental evidence suggests that diminished global alpha, and beta powers in neurodegenerative disorders such as Parkinson’s disease (PD), could be restored to optimal levels following multisensory neurorehabilitation20, or after dopamine replacement therapy, presenting the possibility that these OBRs could serve as putative biomarkers4,6,11,27,32,38,39. Although current EEG literature is somewhat heterogeneous in the measurement and the role of OBRs in neural communication17, a coherent body of evidence is emerging that suggests these oscillations are not just the byproduct of action potentials, but also play a role in inter-network communication and time-dependent processes in the brain16,34. The purpose of this mini review is to provide evidence for the potential use of frequency-specific OBRs as biomarkers that may have applications for the delivery of targeted therapies in neurodegenerative disorders, primarily in PD.
EEG Changes in Motor Learning, Task Mastery, and Skill Retention
At baseline, during consciousness and in the absence of external stimulation also referred to as a resting state, alpha frequencies remain relatively low or synchronized; this is associated with a relatively higher power compared to that which occurs while attending to a stimulus or performing a task, where a sudden allocation of cognitive demand or neural resources causes higher alpha oscillations or desynchronization (decreased power)22,26,44. Beta oscillations follow the same pattern at rest, and change abruptly before and after motor performance, but are highly location specific during voluntary movements43. Literature in motor learning and imagery has confirmed a higher alpha synchronization at baseline before observation or execution of a motor task, and desynchronization during action observation or execution of a motor task30,34. Beta synchronization usually occurs abruptly after a motor action, while desynchronization is the dominant pattern during a motor action14,30,35. Di Nota and colleagues (2017), from our group, showed that young ballet-dancers have higher alpha and beta powers while engaged in action observation including 8-second video clips of choreographed ballet dance, and more detailed visual imagery (as measured by the visualized movements included in the vividness of movement imagery questionnaire) in comparison with non-ballet dancers and non-dancers respectively22. These results could be interpreted in light of the truism “practice makes perfect”; practice-related motor mastery lowers the neural demand required for future observation, visualization, and execution of the same task in the real-world studio, which may reflect a cortical to subcortical consolidation, as the task becomes a motor habit1,7,14,22. These findings are also further expanded by an fMRI study by Li et al. (2015), which included 28 dancers and 33 non-dancers and confirmed a marked enhancement of functional connectivity in cortico-basal ganglia loops using fMRI36, which could be the underlying circuit the OBRs use during learning of real world movements through space.
Interestingly, decreased modulation of alpha and beta power, and changes in connectivity between the basal ganglia and the motor cortex are common epiphenomena in aging, and neurodegenerative disorders, and are directly linked to motor performance2,49. Neurorehabilitation outcomes are drastically poor in these patient populations, and the carryover is mainly limited to hours or few days1,45. Given the underlying physiological changes prior to, or during the onset of symptoms, frequency-specific OBRs have been widely studied as both potential culprits and markers of faulty networks, and a closer look at these oscillations could aid in identifying mechanisms involved in the pathogenesis of such disorders11, 32, 39.
Oscillatory Brain Rhythms, Mirror Neurons, and the Default Mode Network
A prime example of the role of OBRs in motor performance is the Rolandic alpha (8-13 Hz), which is a unique frequency band that is mainly recorded over the sensorimotor regions in humans (as opposed to frontal, occipital, or global alpha)39, 44. ERD in this frequency band is thought to be correlated with action observation, and motor preparation, and is considered by many to play a role in the proposed human mirror neuron system (MNS)13,14, 23,31. Braadbaart and colleagues (2013) tested 16 male participants using a manual imitation paradigm during an ongoing EEG-fMRI study, and their results confirmed the role of alpha desynchronization in motor imagery, and action preparation, corresponding to the putative MNS regions13. In addition, Brihmat et al. (2017) tested the possibility of involvement of both the MNS, and the default mode network (DMN) using virtual reality for action observation, and imitation of virtual hand movements while subjects were in an fMRI scanner. They found that the imitation of a motor task could modulate the activation of specific areas including those belonging to the MNS and the DMN14. These findings suggest that frequency-specific oscillations could provide information on network-specific changes during motor learning and performance.
The supporting evidence from developmental studies also suggests that complex tasks such as walking and fine motor movements are encoded in the DMN during the first few years of development, solidifying motor networks for effortless locomotion through the environment and allowing vital sensory and perceptual tasks such as vigilance and scanning to be processed by regions further up in the hierarchy of cognitive resources18, 25. However, deterioration in these networks due to pathology and aging may result in diminished ability of older adults, people with neurodegenerative disorders, and stroke patients to perform sub-optimally on reaction time studies, indicating a lower processing speed48. It seems as though in the presence of neuropathology, basic functions such as gait, posture, memory recall, selective attention and dual-task performance that were once effortless, now require increased neural resources and cognitive load49. As Santiago Ramón Cajal wrote in 1885 “all of the various conformations of the neuron and its various components are simply morphological adaptations governed by the laws of conservation for time, space and material” and as the fundamental physiological behaviour of the brain implies, the brain is on a constant endeavor to find the most cost-effective ways to process information, and tasks associated with higher cognitive demand could slow the rate of processing3. This view is in line with the contemporary explanations for efficient resource allocation in motor task mastery such as the neural efficiency hypothesis22, 43.
The Neural Efficiency Hypothesis Model and Neurodegenerative Disorders
Optimal levels of alpha and beta frequency bands observed in the absence of pathology, and the role of alpha and beta ERS and ERD in motor learning and competency suggests that the neural efficiency hypothesis could explain changes in motor behaviour in both health and disease6,15,44. In neurodegenerative disorders, an impediment in an optimal shift in neural resources is thought to be one of the main factors associated with changes in sensory, motor, and cognitive performance24, 26. In humans, the task of walking occurs with minimal shifts in attentional resources, in fact, we can walk, carry an object, and watch for environmental hazards while talking to a friend5. Gait analysis studies have demonstrated the intricate step-wise mechanism of ambulation, where agonist and antagonist muscles are contracted and relaxed harmoniously in a matter of milliseconds, and changes in the center of mass would occur in a rhythmic manner after each step, while vestibular and proprioceptive feedbacks act as modulators between the muscular, peripheral and the central nervous systems5,12,23. In movement disorders or stroke, patients are required to make deliberate shifts of attention, when performing previously learned motor tasks (e.g. walking or movement in affected extremity) which in turn tax the available resources, causing an effortful and inaccurate production of movement as well as interference in other cognitive processes (e.g., inability to multitask while standing or walking)48. Motor dysfunction is not just limited to movement disorders, but has been observed in other neurodegenerative processes, particularly in preclinical stages of Alzheimer’s disease (AD)2, 26. As mentioned earlier, the role of the DMN in task mastery, and decoupling of this network from regions involved in high cognitive load during motor performance of previously learned tasks resembles alpha and beta ERS/ERD associated with the network-specific allocation of neural resources in learned vs. novel tasks (Figure 1). This model could explain complications that arise from motor and cognitive changes in neurodegenerative disorders.
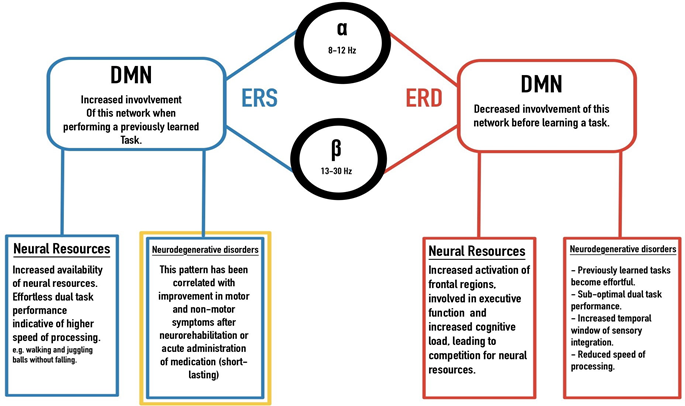
Figure 1: A model based on similarities and overlap of alpha and beta oscillatory behaviour and the DMN: ERS/ERD in alpha and beta frequency bands indicating network changes in competent vs. novice studies. Increased involvement of the DMN, sparing resource-intensive regions in the frontal area during effortless execution of a motor task, resembles ERS and ERD patterns. These results have also been observed in neurodegenerative disorders, where previously effortless tasks require a higher neural demand leaving limited resources for other demanding cognitive tasks. The gold box indicates an optimal goal in targeted therapies.
Amelioration of Motor and Cognitive Symptoms, and Bottom-Up Modulation of the Oscillatory Brain Rhythms
As discussed in the previous section, learning and practice promote efficient resource allocation in healthy individuals, while the presence of pathology disrupts this efficiency; rhythmic exogenous stimuli appear to influence endogenous processes in neurodegenerative disorders, specifically in PD42, 47. In 1871 the Parisian neurologist, Jean-Martin Charcot observed, that the motor symptoms of patients with PD (PwPD) improved after a train or horseback ride; this observation led him to build his “fauteuil trépidant” or shaking chair to replicate the results of his observations29. Although amelioration of symptoms was achieved after using the shaking chair, results were not long lasting and eventually, he abandoned it. Almost a century later, the use of auditory cues (music) and metronome therapy became popular as rehabilitation interventions, and these have since shown to be effective sensory entrainment methods in gait retraining for PwPD (Figure 2)9,10,28,42,46,47.
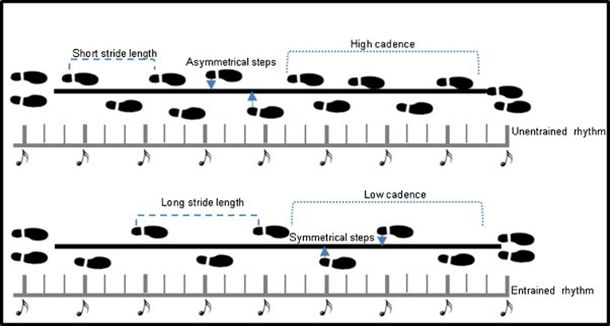
Figure 2: An example of gait pattern before and after training with auditory stimuli: Nombela et al (2013)
PwPD who were gait re-trained with rhythmic musichad symmetrical and longer strides as well as higher cadence41. Later, visual cues (straight lines) were added on the floor while the metronome beats played, and improvements in quality and speed of gait were superior in comparison with the auditory-cue only condition5,42. Although these observational studies clearly showed that external cues could change the quality of gait and movement, the mechanism of these changes was neither explored nor explained. In our lab, Levkov et al (2014) explored the impact of multisensory motor training in PwPD attending the dance with PD (DwP) classes where the amalgamation of audio, visual, tactile, and motor cues provide an enriched exogenous tuning mechanism. Based on resting state EEG results, a dance-induced frontal alpha synchronization was observed35. Earlier, in the same cohort, during the first year of our research program on DwP, Bearss et al (2017) showed a marked improvement on the Berg Balance Scale, and gait speed after 12 weeks of participation in DwP classes8. These results support the notion that exogenous cues (stimuli) could potentially change frequency-specific OBRs linked with affected networks in neurodegenerative disorders1, 10. Another clear example of such bi-directionality is provided by Li-Huei Tsai’s lab at MIT, where the properties of gamma frequency bands (20-50 HZ) are used to design a non-invasive 40 Hz flickering light regime, that reduced plaque formation in a mouse model of Alzheimer’s. These results are significant, as bottom-up approaches such as flickering lights were shown to change not only the OBRs, but also the biochemistry of the mouse brain33. Moving from multisensory training to a pharmacological approach or chemical entrainment, Melgari et al. (2014) investigated the effects of acute administration of L-dopa one alpha and beta powers in 24 PwPD 60 minutes after oral intake. Their results showed amelioration of motor symptoms measured on the motor component of the Unified PD Rating Scale (UPDRS III), followed by a significant increase in alpha power spectrum over the central temporo-parietal regions, and beta power in the central parietal regions38. These results are complimentary with an fMRI study done by Gao et al (2017) where they compared the functional connectivity of basal ganglia of 30 PwPD before and after L-dopa administration where the results confirmed the normalization of the motor pathways of the basal ganglia, supporting the specificity of the OBRs in quantifying changes in putative neural networks27. The above results show a clear correlation between amelioration of symptoms, and the recorded cortical frequency-specific oscillations as well as inter/intra-network connectivity. Whether OBRs and functional connectivity changes are due to multisensory training or pharmacological agents, these results support the hypothesis that these oscillations accurately quantify the cortical mechanisms of symptom deterioration or amelioration at the neurophysiological level.
Discussion
Oscillatory brain rhythms have been shown to be promising biomarkers for neurodegenerative disorders. If we accept the bidirectional properties of these oscillations and consider the role of bottom-up tuning or entrainment, therapies and neurorehabilitation models utilizing rhythmic frequency-specific audio, visual, vestibular and tactile stimuli such as dance could potentially change underlying neural networks leading to an enhanced quality of life and a slower rate of disease progression. The current evidence discussed in this paper suggests that casting a broad net, as in the case of multisensory approaches, may be the most successful rehabilitation model to date, which updates previous models by taking OBRs into consideration21. In medicine, broad-spectrum antibiotics normally treat infections caused by persistent or unknown bacterial pathogens; here, multisensory motor training provides a bottom-up action/perception approach, targeting a wide array of frequency-specific oscillations affected by neurodegenerative processes. Based on current literature and anecdotal evidence, we argue that the potential use of the OBRs as biomarkers in the context of neurofeedback and in the delivery of network-specific therapies could increase specificity and effectiveness of neurorehabilitation interventions.
Acknowledgment
Special thanks to our PD participants and JoeLab members for their tireless efforts to advance the knowledge of PD. Funding for this project was from National Science and Engineering Research Council (NSERC) Discovery grant to JFXD and donation from the Irpinia Club of Toronto to JFXD, and Parkinson’s Society Canada pilot grant to JFXD.
References
- Abbruzzese G, Avanzino L, Marchese R, et al. Action Observation and Motor Imagery: Innovative Cognitive Tools in the Rehabilitation of Parkinson’s Disease. Parkinson’s Dis. 2015; 124214. https://doi.org/10.1155/2015/124214
- Alexander DM1, Arns MW, Paul RH, et al. EEG markers for cognitive decline in elderly subjects with subjective memory complaints. J Integr Neurosci. 2006; 5: 49–74. https://doi.org/10.1142/S0219635206001021
- Andres-Barquin PJ. Ramón y Cajal: a century after the publication of his masterpiece. Endeavour. 2001; 25; 13-17.
- Androulidakis AG, Mazzone P, Litvak V, et al. Oscillatory activity in the pedunculopontine area of patients with Parkinson’s disease. Exp Neurol. 2008; 211: 59–66. https://doi.org/10.1016/j.expneurol.2008.01.002
- Azulay JP, Mesure S, Blin O. Influence of visual cues on gait in Parkinson’s disease: contribution to attention or sensory dependence? J Neurol Sci. 2006; 248: 192–195. https://doi.org/10.1016/j.jns.2006.05.008
- Babiloni C, Del Percio C, Vecchio F, et al. Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clinical Neurophysiology. 2016; 127: 641–654. https://doi.org/10.1016/j.clinph.2015.04.068
- Bar RJ, DeSouza JFX. Tracking Plasticity: Effects of Long-Term Rehearsal in Expert Dancers Encoding Music to Movement. PLOS ONE. 2016; 11: e0147731.https://doi.org/10.1371/journal.pone.0147731
- Bearss KA, McDonald KC, Bar RJ, et al. Improvements in balance and gait speed after a 12 -week dance intervention for Parkinson’s disease. Advances in Integrative Medicine. 2017; 4: 10–13. https://doi.org/10.1016/j.aimed.2017.02.002
- Bella SD, Benoit CE, Farrugia N, et al. Gait improvement via rhythmic stimulation in Parkinson’s disease is linked to rhythmic skills. Sci Rep. 2017; 7. https://doi.org/10.1038/srep42005
- Bellinger D, Altenmüller E, Volkmann J. Perception of Time in Music in Patients with Parkinson’s Disease–The Processing of Musical Syntax Compensates for Rhythmic Deficits. Front Neurosci. 2017; 11. https://doi.org/10.3389/fnins.2017.00068
- Bergman H, Deuschl G. Pathophysiology of Parkinson’s disease: from clinical neurology to basic neuroscience and back. Mov Disord. 2002; 17 Suppl 3: S28-40.
- Blin O, Ferrandez AM, Serratrice G. Quantitative analysis of gait in Parkinson patients: increased variability of stride length. J Neurol Sci. 1990; 98: 91–97.
- Braadbaart L, Williams JH, Waiter GD. Do mirror neuron areas mediate mu rhythm suppression during imitation and action observation? International Journal of Psychophysiology. 2013; 89: 99–105. https://doi.org/10.1016/j.ijpsycho.2013.05.019
- Brihmat N, Tarri M, Quidé Y, et al. Action, observation or imitation of virtual hand movement affect differently regions of the mirror neuron system and the default mode network. Brain Imaging Behav. 2017. https://doi.org/10.1007/s11682-017-9804-x
- Brittain JS, Brown P. Oscillations and the basal ganglia: Motor control and beyond. Neuroimage. 2014; 85: 637–647. https://doi.org/10.1016/j.neuroimage.2013.05.084 353
- Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013; 80: 751–764. https://doi.org/10.1016/j.neuron.2013.10.002
- Buzsáki G, Watson BO. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci. 2012; 14: 345–367.
- Chang CL, Kubo M, Buzzi U, et al. Early Changes in Muscle Activation Patterns of Toddlers During Walking. Infant Behav Dev. 2006; 29: 175–188.https://doi.org/10.1016/j.infbeh.2005.10.001
- Cohen MX. Where Does EEG Come From and What Does It Mean? Trends in Neurosciences. 2017; 40(4): 208-218. doi:10.1016/j.tins.2017.02.004
- DeSouza JFX, Bearss KA. Progression of Parkinson’s disease symptoms halted using dance over 3-years as assessed with MDS-UPDRS. Neuroscience Meeting Planner. San Diego: Society for Neuroscience Abstracts Online. 2018. https://abstractsonline.com/pp8/-!/4649/presentation/22209
- Dhami P, Moreno S, DeSouza JF. New framework for rehabilitation – fusion of cognitive and physical rehabilitation: the hope for dancing. Front Psychol. 2014; 5: 1478. https://doi.org/10.3389/fpsyg.2014.01478
- Di Nota PM, Chartrand JM, Levkov GR, et al. Experience-dependent modulation of alpha and beta during action observation and motor imagery. BMC Neurosci. 2017; 18: 28. https://doi.org/10.1186/s12868-017-0349-0
- Ding C, Palmer CJ, Hohwy J, et al. Parkinson’s disease alters multisensory perception: Insights from the Rubber Hand Illusion. Neuropsychologia. 2017; 97: 38–45. https://doi.org/10.1016/j.neuropsychologia.2017.01.031 386
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol. 2013; 7: 193–224. https://doi.org/10.1111/jnp.12028
- Fransson P, Skiöld B, Horsch S, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007; 104: 15531– 392 15536. https://doi.org/10.1073/pnas.0704380104
- Friston KJ, Bastos AM, Pinotsis D, et al. LFP and oscillations—what do they tell us? Current Opinion in Neurobiology, SI: Brain rhythms and dynamic 396 coordination. 2015; 31: 1–6. https://doi.org/10.1016/j.conb.2014.05.004
- Gao LL, Zhang JR, Chan P, et al. Levodopa Effect on Basal Ganglia Motor Circuit in Parkinson’s Disease. CNS Neurosci Ther. 2017; 23: 76–86. 400 https://doi.org/10.1111/cns.12634
- Ghai S, Ghai I, Schmitz G, et al. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci Rep. 2018; 8: 506. https://doi.org/10.1038/s41598-017-16232-5
- Goetz CG. The History of Parkinson’s Disease: Early Clinical Descriptions and Neurological Therapies. Cold Spring Harb Perspect Med. 2011. 1.https://doi.org/10.1101/cshperspect.a008862
- Gómez-Ramírez J, Freedman S, Mateos D, et al. Exploring the alpha desynchronization hypothesis in resting state networks with intracranial electroencephalography and wiring cost estimates. Sci Rep. 2017; 7. https://doi.org/10.1038/s41598-017-15659-0
- Hobson HM, Bishop DVM. Mu suppression – A good measure of the human mirror neuron system? Cortex. 2016; 82: 290–310. https://doi.org/10.1016/j.cortex.2016.03.019 417
- Hou Y, Yang J, Luo C, et al. Dysfunction of the Default Mode Network in Drug-Naïve Parkinson’s Disease with Mild Cognitive Impairments: A Resting-State fMRI Study. Front Aging Neurosci. 2016; 8. https://doi.org/10.3389/fnagi.2016.00247
- Iaccarino HF, Singer AC, Martorell AJ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016; 540: 230–235. https://doi.org/10.1038/nature20587
- Klassen BT, Hentz JG, Shill HA, et al. Quantitative EEG as a predictive biomarker for Parkinson disease dementia. Neurology. 2011; 77(2): 118-124. doi:10.1212/WNL.0b013e318224af8d
- Levkov GR, Di Noto PM, Montefusco-Siegmund R, et al. Global alpha slowing in individuals with Parkinson’s disease and dance-induced increases in frontal alpha synchronization. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience Abstracts. 2014; 437.
- Li G, He H, Huang M, et al. Identifying enhanced cortico-basal ganglia loops associated with prolonged dance training. Sci Rep. 2015; 5: 10271. https://doi.org/10.1038/srep10271
- Lucas M, Chaves F, Teixeira S, et al. Time perception impairs sensory-motor integration in Parkinson’s disease. Int Arch Med. 2013; 6: 39. https://doi.org/10.1186/1755-7682-6-39
- Melgari JM, Curcio G, Mastrolilli F, et al. Alpha and beta EEG power reflects L-dopa acute administration in parkinsonian patients. Front Aging Neurosci. 2014; 6. https://doi.org/10.3389/fnagi.2014.00302
- Miller RA, Thaut MH, McIntosh GC, et al. Components of EMG symmetry and variability in parkinsonian and healthy elderly gait. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control. 1996; 101: 1–7. https://doi.org/10.1016/0013-4694(95)00209-X
- Nelson AB, Moisello C, Lin J, et al. Beta Oscillatory Changes and Retention of Motor Skills during Practice in Healthy Subjects and in Patients with Parkinson’s Disease. Front Hum Neurosci. 2017; 11: 104. https://doi.org/10.3389/fnhum.2017.00104
- Nishikawa N, Shimo Y, Wada M, et al. Effects of Aging and Idiopathic Parkinson’s Disease on Tactile Temporal Order Judgment. PLOS ONE. 2015; 10: e0118331. https://doi.org/10.1371/journal.pone.0118331
- Nombela C, Hughes LE, Owen AM, et al. Into the groove: Can rhythm influence Parkinson’s disease? Neuroscience & Biobehavioral Reviews. 2013; 37: 2564–2570.https://doi.org/10.1016/j.neubiorev.2013.08.003
- O’Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther. 2002; 82: 888–897.
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999; 110: 1842–1857.
- Shine JM, Matar E, Ward PB, et al. Differential neural activation patterns in patients with Parkinson’s disease and freezing of gait in response to concurrent cognitive and motor load. PLoS ONE. 2013; 8: e52602. https://doi.org/10.1371/journal.pone.0052602
- Thaut MH, McIntosh GC, Rice RR, et al. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Movement Disorders. 11: 193–200. https://doi.org/10.1002/mds.870110213
- van Wegen E, de Goede C, Lim I, et al. The effect of rhythmic somatosensory cueing on gait in patients with Parkinson’s disease. J Neurol Sci. 2006; 248: 210–214. https://doi.org/10.1016/j.jns.2006.05.034
- Westlake KP, Hinkley LB, Bucci M, et al. Resting State Alpha-band Functional Connectivity and Recovery after Stroke. Exp Neurol. 2012; 237: 160–169. https://doi.org/10.1016/j.expneurol.2012.06.020
- Zappasodi F, Marzetti L, Olejarczyk E, et al. Age-Related Changes in Electroencephalographic Signal Complexity. PLoS One. 2015; 10. https://doi.org/10.1371/journal.pone.0141995
