Interactions between genetic and environmental factors and schizophrenia: Insights from KPNA1-deficient mice
Hirotaka Nomiya1, Masami Yamada1,2*
1Department of Cell Biology and Biochemistry, Division of Medicine, Faculty of Medical Sciences, University of Fukui, Matsuoka Shimoaizuki, Eiheiji-cho, Yoshida-gun, Fukui, Japan
2Life Science Innovation Center, University of Fukui, Bunkyo, Fukui-City, Fukui, Japan
Abstract
The interactions between genetic and environmental factors (G x E interactions) play a crucial role in the pathogenesis of schizophrenia. The administration of phencyclidine, a psychotropic drug, to Kpna1-deficient mice induces behavioral abnormalities resembling schizophrenia. In the nucleus accumbens of these mice, the expressions of dopamine receptors, an RNA editing enzyme, and cytoplasmic dynein demonstrate gene-environment interaction-dependent alterations. Kpna1-deficient mice may be useful as a gene-environment interaction model for schizophrenia and provide insights into its pathogenesis. Further, changes in gene expression in the nucleus accumbens may be involved in the development of schizophrenia.
Introduction
Literature Search
Schizophrenia is a complex mental disorder that typically develops in late adolescence or early adulthood. It is characterized by a range of symptoms, including positive symptoms such as hallucinations and delusions, negative symptoms like flattened affect and avolition, and cognitive impairments affecting memory, attention, and executive function. Pharmacological treatment with atypical antipsychotics, including dopamine d2 and serotonin 5-HT2A receptor inhibitors, has been used. However, the mechanism underlying its pathogenesis remains unclear1,2.
Several mice models of schizophrenia have been developed to elucidate the pathogenesis of the disorder. These models can be broadly categorized into genetic (G) and environmental (E) models. Genetic models of representative genes associated with schizophrenia development, including disrupted-in-schizophrenia 1, neuregulin 1, and dystrobrevin-binding protein 1 gene-deficient mice3-6. Environmental models, on the other hand, are generated by exposing animals to factors known to induce schizophrenia-like symptoms, such as the administration of psychotomimetic substances like phencyclidine (PCP), amphetamine, and MK-801, or through isolation stress7-10.
The interaction between genetic and environmental factors (G × E) has recently been suggested to have a significant impact on the pathogenesis of psychiatric disorders11,12. Examination of the exomes of schizophrenia patients have implicated mutations of human importin α5 (mouse importin α1; gene symbol: Kpna1; protein symbol: KPNA1) in psychiatric disorders13. The usefulness of mouse models with KPNA1 as a genetic factor and social isolation or PCP as an environmental factor has been reported14,15.
The purpose of this mini review is to present insights into the usefulness of the G x E mouse model for investigating the pathogenesis and progression of schizophrenia, as well as its underlying molecular mechanisms.
KPNA1 and Schizophrenia
KPNA1 is a member of the importin α family, which assists in the transport of proteins from the cytoplasm to the nucleus in eukaryotes. Importin α recognizes classical nuclear localization signals, which are composed of basic amino acid clusters, and forms a trimeric complex with importin β that is transported into the nucleus via the nuclear pore complex. In the central nervous system, KPNA1 is the most abundantly expressed member of the importin α family16. It is an important regulator of neuronal development in mice embryonic stem cells17. Kpna1 knockout (KO) mice (also known as Importin α5 KO mice from the human nomenclature) have demonstrated psychiatric disorder-related behavioral deficits such as a prominent reduction in anxiety-like behaviors and reduced acoustic startle response14,18. The KPNA1 mutations identified in patients with schizophrenia are located outside the conventional NLS recognition region, implying that KPNA1 plays a role in schizophrenia development via mechanisms other than nucleocytoplasmic transport13,19.
Our G x E mouse model
Previous research investigating the interaction between genetic and environmental factors utilized a three-week environmental stress period administered during adolescence (ages 5–8 weeks)20,21. Among these studies, the first week (age 5 weeks) was identified as the critical period of susceptibility to stress during adolescence. To target the critical period of vulnerability to environmental stress using PCP as a stress factor, we subcutaneously administered 10 mg/kg/day of PCP to 5-week-old male Kpna1 KO and WT mice for 7 consecutive days (Figure 1a). Behavioral tests were conducted after the mice reached 8 weeks of age. The vehicle (saline) was administered to the control group. Brain tissue was extracted from mice at 10 weeks of age after behavioral testing, and gene expression analysis was subsequently conducted. We found that subchronic administration of phencyclidine, a psychotropic drug, induced vulnerability and behavioral abnormalities consistent with schizophrenia symptoms in Kpna1-deficient mice. Microarray assessment revealed that the levels of expression of dopamine d1/d2 receptors, an RNA editing enzyme, and a cytoplasmic dynein component demonstrated significant gene-environment (G × E) interaction-dependent alterations in the nucleus accumbens (NAc) (Figure 1b). Our findings demonstrate that Kpna1-deficient mice may be useful as G × E interaction models for psychiatric disorders and further investigation of their pathogeneses.
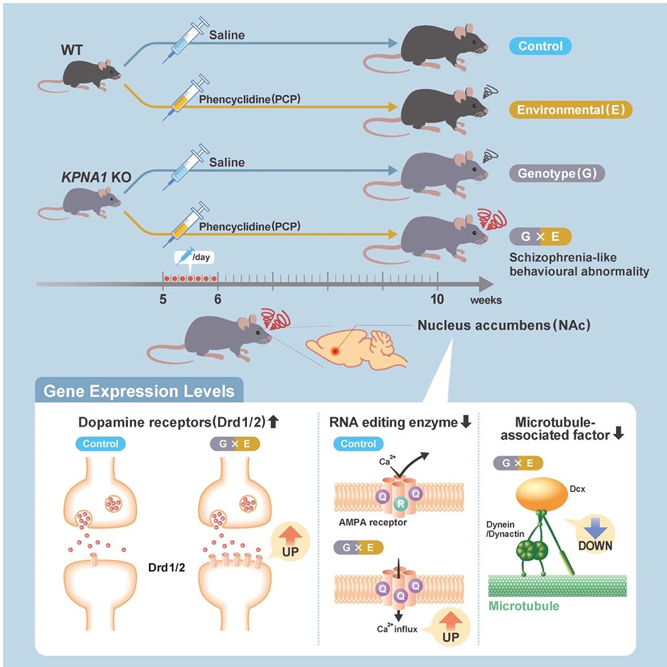
Figure 1: Graphical abstract of our G x E model mice study findings.
We found that subchronic administration of phencyclidine induced vulnerability and behavioral abnormalities consistent with the symptoms of schizophrenia in Kpna1-deficient mice. Microarray assessment revealed that the levels of expression of dopamine d1/d2 receptors, an RNA editing enzyme, and a cytoplasmic dynein component demonstrated significant gene-environment (G × E) interaction-dependent alterations in the NAc. Our findings demonstrate that Kpna1-deficient mice may be useful as G × E interaction mice models for psychiatric disorders and further investigations into the pathogenesis of such diseases and disorders. NAc: nuclear accumbens, Dcx: doublecortin X.
Significance of the NAc in Schizophrenia
The cortico-basal ganglia-thalamus-cortical circuit is well-recognized for its significant involvement in motor control, decision-making, and cognitive activities22. Deficiencies in this network have been attributed to various movement disorders and mental conditions. Consequently, we conducted gene expression analysis of the prefrontal cortex (PFc) and nucleus accumbens (NAc).
RNA extraction from brain tissue was performed. Utilizing the Clariom S mouse assay and the GeneChip microarray analysis system GCS 3000Dx, microarray analysis was conducted. Robust multichip analysis was employed to normalize the data. In order to identify differentially expressed genes (DEGs) between two groups, a fold change factor greater than 1.7 and a p-value less than 0.05, as determined by Welch's t-test, were required. Benjamini's method was utilized to compute the false discovery rate (FDR) in order to account for the influence of multiple testing. Pathway and Gene Ontology (GO) analyses were performed utilizing the DEGs identified, in conjunction with a range of bioinformatics tools, such as DAVID, GSEA, and Metascape23-25. Our comprehensive genetic analysis revealed marked differences in gene expression between the PFc and NAc (Figure 2). Different brain regions express the genes, and we observed differences in their levels of expression. Overall, we identified more DEGs in the NAc than in the PFc; for example, we identified fewer DEGs in the PFc than in the NAc on comparing the PCP-treated groups. These findings demonstrated that genetic and environmental factors have distinct effects on various brain regions (Figure 3). While several DEGs in the NAc were shared by the PCP-treated groups, a considerable number of DEGs belonging to the same ontology but having differential expressions were identified (Figure 4). GO enrichment analysis was applied to the differences in the DEGs in the NAc of the PCP-treated groups to extract the "biological meanings”, which revealed fluctuations in the expression of factors related to AMPA receptor activation and calcium ion influx (Figure 5). In addition, the dopamine receptors d1/d2 were significantly up-regulated.
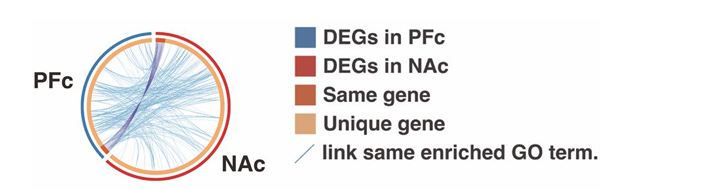
Figure 2: Differences in gene expressions of the PFc and NAc and the related functional factors in each group.
Circos plot shows how the DEGs overlap. Dark orange color represents the genes that are shared by multiple lists, while the light orange color represents unique DEGs. Purple lines link the same gene that are shared by multiple DEGs. Blue lines link the genes, although different, fall under the same ontology term. DEGs: differentially expressed genes, PFc: prefrontal cortex, NAc: nuclear accumbens.
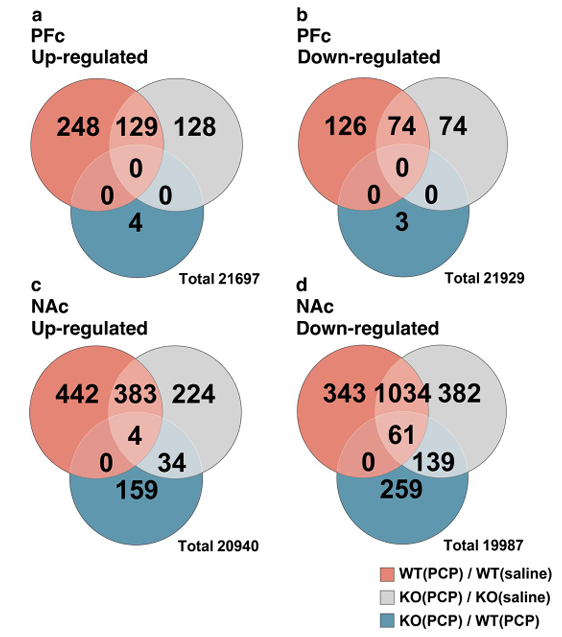
Figure 3: The number of differentially expressed genes in each group and a Venn diagram.
Genes with fold change greater than or less than 1.7 times and p < 0.05 in the Welch's T-test were selected as DEGs. (a) Up-regulated DEGs in the PFc of each group. (b) Down-regulated DEGs in the PFc of each group. (c) Up-regulated DEGs in the NAc of each group. (d) Down-regulated DEGs in the NAc of each group. The DEGs obtained by comparing the expression variations of WT(PCP) and WT (saline) are labeled as WT(PCP)/WT (saline). NAc contained more DEGs than the PFc in the KO(PCP) than in WT(PCP) group. DEGs: differentially expressed genes, PFc: prefrontal cortex, NAc: nuclear accumbens.
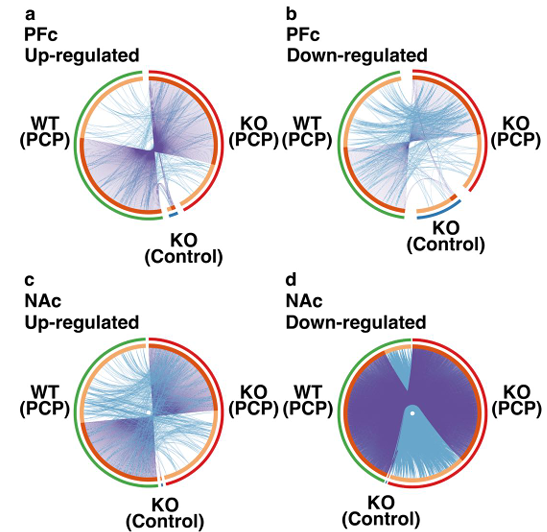
Figure 4: Differences in gene expression in the PFc and NAc and the functional factors in each group.
(a) Up-regulated DEGs in the PFc. (b) Down-regulated DEGs in the PFc. (c) Up-regulated DEGs in the NAc. (d) Down-regulated DEGs in the NAc. Circos plot shows how DEGs in the NAc and PFc overlap. Dark orange color represents the genes that are shared by multiple lists and the light orange color represents the unique DEGs. Purple lines link the genes that are shared by multiple DEGs. Blue lines link the genes that fall under the same ontology term although they are different. DEGs: differentially expressed genes, PFc: prefrontal cortex, NAc: nuclear accumbens.
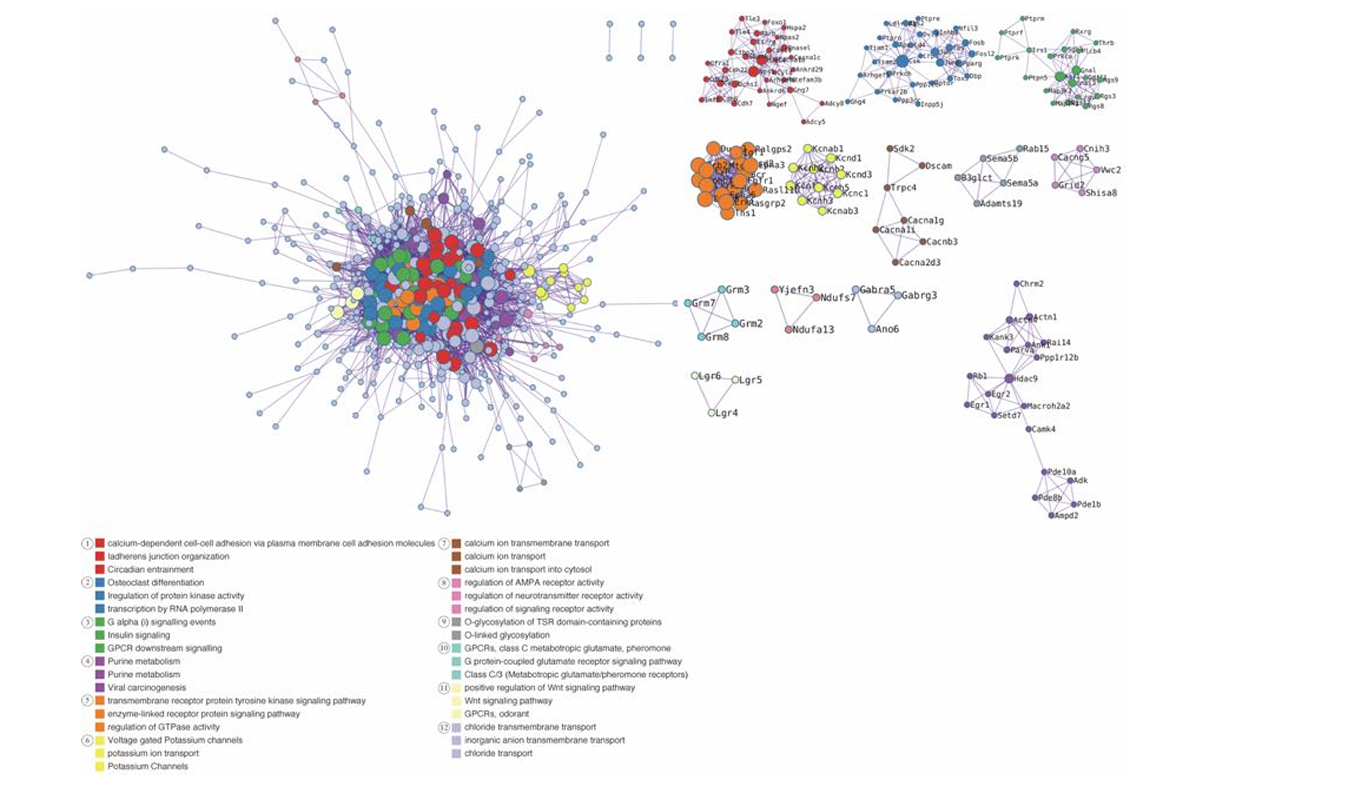
Figure 5: All protein-protein interaction networks for genes showing higher transcription counts in the NAc of the PCP-treated groups.
A network architecture was generated from a subset of representative terms that were extracted from the entire cluster. To be precise, every term is represented by a circle node, the size of which correlates with the number of input genes associated with that term. Additionally, the color of the circle node indicates its cluster identity, with nodes of identical color indicating membership in the same cluster. An edge connects terms that have a similarity score greater than 0.3; the thickness of the edge represents the similarity score. A term is selected from each cluster to have its corresponding term description displayed as a label. GO: gene ontology, PPI: protein-protein interactions, NAc: nuclear accumbens.
Overactivity of dopamine in the mesolimbic system is a major hypothesis for the etiology of schizophrenia26,27. Increased Drd2 density in the striatum contributes to the development of schizophrenia28,29. Thus, we hypothesized that behavioral abnormalities reminiscent of schizophrenia observed in our mouse model were related to changes in Drd2 expression.
According to previous reports, patients with schizophrenia have a dysfunctional α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors30,31; however, the mechanism underlying the contributions of AMPA receptors to the pathogenesis of schizophrenia is not yet understood. In the NAc, we observed altered expressions of an RNA-editing enzyme adenosine deaminases acting on RNA (ADAR), an editing enzyme of the AMPA receptor subunits GRIA2/GluR232. It has been reported that the Q/R site of GRIA2 is 100% edited under normal conditions, while, unedited GRIA2 Q/R sites increase intracellular Ca2+ influx33. In our mouse model, ADAR2 expression was reduced by 0.48-fold, whereas GRIA2 expression was elevated by 1.97-folds compared to control mice in the NAc, indicating that an increase in unmodified GRIA2 leads to an increase in Ca2+ influx. Our gene expression analysis suggested that AMPA receptor dysfunction due to abnormal RNA editing may be involved in the pathogenesis of schizophrenia.
In the neural circuitry of the brain, the NAc receives projections from the PFc via the glutamatergic neurons. The NAc is a site for dopaminergic modulation of neurotransmission and may be prone to the dysregulation of genes characteristic of schizophrenia34. It has also been reported that chronic dysregulation of the NAc triggers altered gene expression in the PFc35. If our model reflects the early onset of schizophrenia, then it is possible that the development of schizophrenia originates in the NAc. The enriched functions of G × E-interacting genes were different in the PFc and NAc. Additionally, enrichment in the NAc was associated with behavioral abnormalities, suggesting that increased vulnerability to PCP in the NAc following Kpna1 KO is involved in the development of schizophrenia. Environmental stress was introduced to our mouse model for a brief period, coinciding with the onset of adolescence, which may indicate susceptibility to environmental stress during specific phases of neurodevelopment. We have demonstrated that KPNA1 is implicated in axonal transport36. Subunits of dopamine and glutamate receptors are reportedly transported via axons by microtubule motors; therefore, disruption of axonal transport in the NAc may play a role in the development of schizophrenia37. Although this characteristic has hitherto remained unexplored in the context of schizophrenia pathogenesis, it has emerged as a pivotal hypothesis in the study of the disorder.
Conclusions
Our Kpna1-deficient psychotropic drug-induced schizophrenia model offers a robust platform for investigating G × E interactions in the context of schizophrenia. The findings presented in this study contribute to our understanding of the molecular mechanisms underlying the pathogenesis of schizophrenia and may guide the development of targeted therapeutic interventions. Future research should focus on further elucidating the role of the NAc in the progression of schizophrenia and exploring the potential of KPNA1 as a novel therapeutic target.
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this mini review.
Ethics Declarations
In accordance with ethical standards and guidelines for the use of animals in research, the animal experiments conducted in this study were approved by the Animal Care and Use Committees of the University of Fukui. All procedures involving animals were performed in compliance with relevant laws and regulations, and every effort was made to minimize any potential discomfort or distress to the animals. Prior to the commencement of the study, informed consent was obtained from the respective authorities, and proper measures were taken to ensure the humane and ethical treatment of the animals throughout the duration of the experiments.
Acknowledgements
We would like to express our gratitude to Koki Sakurai, Takatoshi Hikida, and Yoshihiro Yoneda at Osaka University, as well as Yoichi Miyamoto and Masahiro Oka at the National Institutes of Biomedical Innovation, Health and Nutrition. This work was supported by the following funding sources: the Hoansha Foundation Research Grant, Naito Foundation Research Grant, Takeda Foundation Visionary Research Grant (Step), Uehara Memorial Foundation Research Grant, Terumo Life Science Foundation Research Grant, and Organization for Life Science Advancement Programs at the University of Fukui, awarded to M.Y.
References
- Saha S, Chant D, Welham J, et al. A Systematic Review of the Prevalence of Schizophrenia. PLoS Medicine. 2005; 2(5): e141. https://doi.org/10.1371/journal.pmed.0020141
- Gaebel W, Zielasek J. Schizophrenia in 2020: Trends in diagnosis and therapy. Psychiatry and Clinical Neurosciences. 2015; 69: 661-673. https://doi.org/10.1111/pcn.12322
- Hikida T, Jaaro-Peled H, Seshadri S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proceedings of the National Academy of Sciences. 2007; 104: 14501-14506. https://doi.org/10.1073/pnas.0704774104
- Stefansson H, Sigurdsson E, Steinthorsdottir V, et al. Neuregulin 1 and Susceptibility to Schizophrenia. The American Journal of Human Genetics. 2002; 71: 877-892. https://doi.org/10.1086/342734
- Takao K, Toyama K, Nakanishi K, et al. Impaired long-term memory retention and working memory in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Molecular Brain. 2008; 1: 11. https://doi.org/10.1186/1756-6606-1-11
- Takao K, Kobayashi K, Hagihara H, et al. Deficiency of Schnurri-2, an MHC Enhancer Binding Protein, Induces Mild Chronic Inflammation in the Brain and Confers Molecular, Neuronal, and Behavioral Phenotypes Related to Schizophrenia. Neuropsychopharmacology. 2013; 38: 1409-1425. https://doi.org/10.1038/npp.2013.38
- Mouri A, Noda Y, Enomoto T, et al. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int. 2007; 51: 173-184. https://doi.org/10.1016/j.neuint.2007.06.019
- Tenn CC, Fletcher PJ, Kapur S. Amphetamine-sensitized animals show a sensorimotor gating and neurochemical abnormality similar to that of schizophrenia. Schizophr Res. 2003; 64: 103-114. https://doi.org/10.1016/s0920-9964(03)00009-4
- Bubeníková-Valesová V, Horácek J, Vrajová M, et al. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008; 32: 1014-1023. https://doi.org/10.1016/j.neubiorev.2008.03.012
- Powell SB, Swerdlow NR. The Relevance of Animal Models of Social Isolation and Social Motivation for Understanding Schizophrenia: Review and Future Directions. Schizophrenia Bulletin. 2023; 49: 1112-1126. https://doi.org/10.1093/schbul/sbad098
- Tsuang MT, Bar JL, Stone WS, et al. Gene-environment interactions in mental disorders. World Psychiatry. 2004; 3: 73-83.
- Wahbeh MH, Avramopoulos D. Gene-Environment Interactions in Schizophrenia: A Literature Review. Genes. 2021; 12: 1850. https://doi.org/10.3390/genes12121850
- Girard SL, Gauthier J, Noreau A, et al. Increased exonic de novo mutation rate in individuals with schizophrenia. Nature Genetics. 2011; 43: 860-863. https://doi.org/10.1038/ng.886
- Sakurai K, Itou T, Morita M, et al. Effects of Importin α1/KPNA1 deletion and adolescent social isolation stress on psychiatric disorder-associated behaviors in mice. PLOS ONE. 2021; 16: e0258364. https://doi.org/10.1371/journal.pone.0258364
- Nomiya H, Sakurai K, Miyamoto Y, et al. A Kpna1-deficient psychotropic drug-induced schizophrenia model mouse for studying gene–environment interactions. Scientific Reports. 2024; 14(1): 3376. https://doi.org/10.1038/s41598-024-53237-3
- Hosokawa K, Nishi M, Sakamoto H, et al. Regional distribution of importin subtype mRNA expression in the nervous system: Study of early postnatal and adult mouse. Neuroscience. 2008; 157: 864-877. https://doi.org/10.1016/j.neuroscience.2008.09.045
- Yasuhara N, Shibazaki N, Tanaka S, et al. Triggering neural differentiation of ES cells by subtype switching of importin-α. Nature Cell Biology. 2007; 9: 72-79. https://doi.org/10.1038/ncb1521
- Panayotis N, Sheinin A, Dagan SY, et al. Importin α5 Regulates Anxiety through MeCP2 and Sphingosine Kinase 1. Cell Reports. 2018; 25: 3169-3179.e3167. https://doi.org/10.1016/j.celrep.2018.11.066
- Jouan L, Girard SL, Dobrzeniecka S, et al. Investigation of rare variants in LRP1, KPNA1, ALS2CL and ZNF480 genes in schizophrenia patients reflects genetic heterogeneity of the disease. Behavioral and Brain Functions. 2013; 9: 9. https://doi.org/10.1186/1744-9081-9-9
- Niwa M, Lee RS, Tanaka T, et al. A critical period of vulnerability to adolescent stress: epigenetic mediators in mesocortical dopaminergic neurons. Hum Mol Genet. 2016; 25: 1370-1381. https://doi.org/10.1093/hmg/ddw019
- Niwa M, Jaaro-Peled H, Tankou S, et al. Adolescent Stress–Induced Epigenetic Control of Dopaminergic Neurons via Glucocorticoids. Science. 2013; 339: 335-339. https://doi.org/10.1126/science.1226931
- Maynard TM, Sikich L, Lieberman JA, et al. Neural development, cell-cell signaling, and the "two-hit" hypothesis of schizophrenia. Schizophr Bull. 2001; 27: 457-476. https://doi.org/10.1093/oxfordjournals.schbul.a006887
- Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nature Communications. 2019; 10(1): 1523. https://doi.org/10.1038/s41467-019-09234-6
- Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005; 102: 15545-15550. https://doi.org/10.1073/pnas.0506580102
- Huang dW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009; 37: 1-13. https://doi.org/10.1093/nar/gkn923
- Bird ED, Spokes EG, Iversen LL. Increased dopamine concentration in limbic areas of brain from patients dying with schizophrenia. Brain. 1979; 102: 347-360. https://doi.org/10.1093/brain/102.2.347
- Conn KA, Burne THJ, Kesby JP. Subcortical Dopamine and Cognition in Schizophrenia: Looking Beyond Psychosis in Preclinical Models. Front Neurosci. 2020; 14: 542. https://doi.org/10.3389/fnins.2020.00542
- Joyce JN, Lexow N, Bird E, et al. Organization of dopamine D1 and D2 receptors in human striatum: receptor autoradiographic studies in Huntington's disease and schizophrenia. Synapse. 1988; 2: 546-557. https://doi.org/10.1002/syn.890020511
- Lee T, Seeman P. Elevation of brain neuroleptic/dopamine receptors in schizophrenia. Am J Psychiatry. 1980; 137: 191-197. https://doi.org/10.1176/ajp.137.2.191
- Zeppillo T, Schulmann A, Macciardi F, et al. Functional impairment of cortical AMPA receptors in schizophrenia. Schizophr Res. 2022; 249: 25-37. https://doi.org/10.1016/j.schres.2020.03.037
- Levite, M. Glutamate receptor antibodies in neurological diseases: Anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: Epilepsy, En. Journal of Neural Transmission. 2014; 121: 1029-1075. https://doi.org/10.1007/s00702-014-1193-3
- Barbon A, Barlati S. Glutamate receptor RNA editing in health and disease. Biochemistry (Moscow). 2011; 76: 882-889. https://doi.org/10.1134/s0006297911080037
- Silberberg G, Lundin D, Navon R, et al. Deregulation of the A-to-I RNA editing mechanism in psychiatric disorders. Hum Mol Genet. 2012; 21: 311-321. https://doi.org/10.1093/hmg/ddr461
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008; 90: 226-235. https://doi.org/10.1016/j.pbb.2008.04.011
- Hikida T, Morita M, Kuroiwa M, et al. Adolescent psychosocial stress enhances sensitization to cocaine exposure in genetically vulnerable mice. Neurosci Res. 2020; 151: 38-45. https://doi.org/10.1016/j.neures.2019.02.007
- Mizuno K, Sugahara M, Kato R, et al. Axonal KPNA1 Signaling Is Involved in the Development of Psychiatric Disorders. Available at SSRN: https://ssrn.com/abstract=4682260
- Alsabban AH, Morikawa M, Tanaka Y, et al. Kinesin Kif3b mutation reduces NMDAR subunit NR2A trafficking and causes schizophrenia-like phenotypes in mice. EMBO J. 2020; 39: e101090. https://doi.org/10.15252/embj.2018101090
