Neural Control and Precision of Spike Phasing in Flight Muscles
Fritz-Olaf Lehmann, PhD
Abstract
Rhythmic locomotor behavior in animals requires exact timing of muscle activation within the locomotor cycle. Neural strategies for timing control that employ higher brain function, however, suffer from synaptic and neural transmission delays, making them inefficient for control of fast-frequent locomotor systems. Evolutionary pressure on muscle timing control is particularly pronounced in flying insects with wing flapping periods of few milliseconds. In these animals, sensory integration is often achieved at the level of the peripheral nervous system, circumventing the central brain and controlling spike activation phases with little delay, rather than muscle spike frequency. This review is engaged in the precision with which flies adjust power output of their flight muscles and highlights the significance of visual and proprioceptive feedback loops for muscle spike control. Recent results suggest that in flies peripheral feedback loops are keys enabling precise heading control and body stability in flight, and potentially similar to the function of local circuits for locomotor control found in the spinal chord of vertebrates.
Precision of locomotor behavior is key to the evolutionary success of animals and humans because motor control is often challenged in tasks with fastidious demands[1]. Successful handling of locomotor tasks, however, is highly prone to neuromuscular noise[2,3]. Receptor noise typically causes alterations in locomotor control, and motor systems thus require elaborated sensory feedback for optimized performance[4]. In monkeys and humans, elevated motor precision is relevant in a large context of various motor behaviors, including goal-directed tasks such as the control of precision grip by fingers and thumbs while lifting weights and grabbing objects with rough or slippery surfaces[5,6]. Other examples comprise equilibrium reflexes during the cortical control of normal gait, precision stepping[7,8], and precision control of trunk movements[1]. Goal-directed tasks require neural forward models which modify inner-loop feedback control systems, while equilibrium reflexes are typically controlled by means of negative feedback loops.
In vertebrates, precision of muscle force strongly depends on the activity of a synergistic ensemble of numerous motor units[8,9]. In invertebrates, by contrast, the number of motor units is typically reduced and all muscle fibers within a single muscle are often simultaneously driven by the same or few motor neurons[10-12]. Due to the reduced number of motor units and the noisiness of the neural pathways, the quality of sensory integration predominately determines how these animals move under unaffected and externally perturbated locomotor conditions. In particular in flight, precision of locomotor behavior is highly relevant for heading and body posture control using equilibrium reflexes. Motor precision in flying animals such as large insects, birds and bats is challenged by comparatively little aerodynamic friction between the surrounding air and body[13]. Although reduced friction reinforces flight maneuverability and aerial agility, it confronts the neuromuscular apparatus of these animals with elevated demands on motor precision for flight stability and steering[14]. Low frictional damping in flight is thus key to the extraordinary aerial performance of flying birds, bats and insects but at the cost of requiring fast and precise visual and proprioceptive feedback-loop systems[15-17].
Insects control locomotor forces at fractions of the typical human sensumotor response time. During maneuvering flight, for example, fruit flies may change wing flapping amplitude every ~5 ms by few degrees[18], while controlling rotational timing of wing motion at the end of each half stroke within less than ~70 µs[19]. To support theses tiny modifications in kinematics, flies have evolved strategies to improve the precision of flight muscle control. A main strategy is the separation of muscle force to power wing flapping (A-IFM, asynchronous indirect flight muscle, Fig. 1A) from a control system (WCM, wing control muscles, Fig. 1E) that modifies power transmission to the wings. This division of labor helps to control wing motion in insect flight systems based on high-frequency mechanical thoracic oscillators because A-IFM power output may change only little in successive 5-10 ms wing stroke cycles. The physiology of A-IFM is similar to the vertebrate heart muscle. Although A-IFM mechanical power output is correlated with intracellular calcium (Fig. 1B-D), the ~1.0 mm long A-IFM fibers mainly contract in response to cyclic stretching. The stretching amplitude of ~20-30 um results from thoracic deformations during wing flapping. Force transmission occurs through a complex wing hinge that features several hard sclerites and soft membranes. During flight, the tiny WCMs exert force on these sclerites, changing the stiffness inside the wing hinge. During wing cleaning behavior and courtship, WCMs may directly move the wings. However, the short locomotor cycle hinders the nervous system to quickly modulate tonic muscle force in both muscle systems by changing muscle spike frequency.
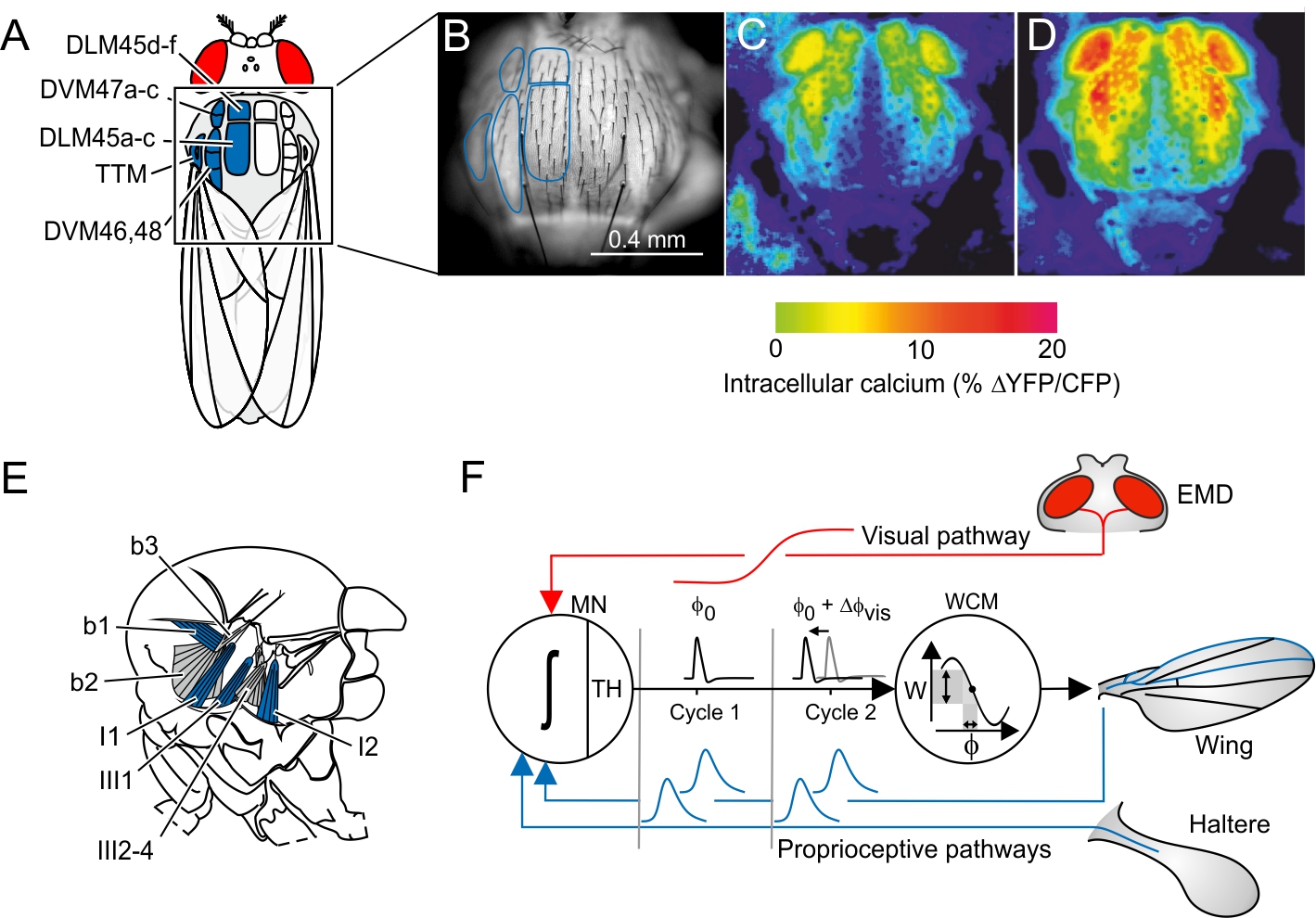
Figure 1: Flight muscle system of flies, calcium-activated flight power muscles (A-IFM), and feedback loop for wing control muscles (WCM). (A) Morphology of A-IFM dorsolongitudinal muscle (DLM, 12 fibers) and dorsoventral muscle (DVM, 14 fibers) inside the fly thorax. TTM, tergo-trochanter muscle. (B) Fluorescence signaling of electrically activated A-IFM expressing the calcium probe Cameleon in a resting (C) and flying fruit fly (D). (E) Major wing control muscles at the fly's wing hinge (b1-3, basalare muscles; I1-2 and III2-4, axillary muscles; side view). (F) Hypothetical feedback loop for activation timing of WCM. Strain-sensitive mechanoreceptors on wings and halteres produce neural spikes (blue) at specific times of the wing stroke cycle (phase-coupled activation). The elementary motion detector (EMD) of the fly's compound eye converts visual motion into graded potentials (red) that are transmitted via descending visual interneurons to the thoracic ganglion (visual pathway). The three inputs are integrated by a WCM motoneuron (MN; ∫, integration process), generating a single muscle action potential at the neuron's threshold (TH) at time Φ0 (activation phase) in each stroke cycle (cycle 1). A change in visual signaling alters the motoneuron's membrane potential and thus delays or advances spike timing (DΦ0) by alterations of depolarization time (cycle 2). Work (W) of WCM changes depending on spike timing because of the muscle’s force-phase curve. The changing work eventually leads to changes in force transmission efficacy and thus wing motion.
Although muscle power depends on neural activation frequency, it also strongly depends on the timing of muscle activation (spike phase) within the locomotor cycle[20-22]. In flying flies, the nervous system provides diverse preferred spike activation phases for the 30 flight control muscles and 26 power muscle fibers, which maximizes their impact on the complex biomechanics of the thoracic flight apparatus[20,23]. Muscle activation phases are phase-locked with the wing stroke cycle during straight flight but they temporally shift (activation phasing) during flight maneuvers[21,23,24]. This spike phasing was also found in other insects such as hawk moths, in which the left-right pairs of flight power muscles precisely fire within 0.5-0.6 ms of each other. This timing difference increases to ~8 ms during turning flight[22,25]. The spike-phasing mechanism in insects provides the nervous system an additional opportunity to influence motor control and locomotor efficacy without changing the cyclic neural activation pattern of the locomotor musculature. Thus in flies, WCMs and A-IFM typically receive not more than a single action potential in every wing stroke cycle[26].
The temporal precision of flight muscle activation in flies results from cyclic proprioceptive feedback generated by force-sensitive gyroscopic halteres and mechanoreceptors (campaniform sensilla) on the wing surface. Halteres are condensed hind wings of the flies’ four-winged ancestors. These sensors produce temporally phase-locked action potentials in every flapping cycle[26]. The feedback tightly links muscle activation phase to the locomotor cycle, with microsecond precision in muscle spike initiation[27,28]. During flight maneuvers and body instabilities, Coriolis forces deflect the halteres, in turn changing timing of proprioceptive feedback and flight muscle tension[29]. Halteres thus act as a gyroscopic system that automatically stabilizes the fly body in flight by phase-induced changes in WCM power[30,31].
Recent studies suggest that phase-locked mechano-sensory feedback and graded visual signaling for flight direction control are integrated by single motoneurons of flight control muscles, circumventing the central brain (CNS)[32]. This local feedback circuitry processes sensory information without much delay since mechanoreceptors and visual motion-sensitive descending interneuron from the CNS constitute rectifying gap junctions on flight muscle motoneurons[33-37]. Visual interneurons deliver timeless cues on the fly’s visual environment by gradually changing their membrane potential of not more than ±5 mV[36-38]. Numerical modeling of the sensory integration process in flies shows that spiking proprioceptive and small graded visual potentials are successfully integrated by single WCM motoneurons (Fig. 1F). The numerical model reproduces multiple experimental findings, offers a mechanistic explanation on gyroscopic body posture control, and explains how vision may change wing kinematics in flies on the cellular level[32].
Precision of locomotor control in vertebrates is typically tuned by motor learning and previous experience[39]. A well-known exception is bird flight that is primarily independent of learning, mainly depending on the development of muscles and neurons[40]. Motor skills in insects, by contrast, are widely recognized as being predominately innate, genetically programmed, fixed-action motor patterns that follow stereotyped rules. An increasing number of recent studies, however, suggest that experience fine-tunes locomotion to a higher precision. This was shown in walking stick insects[41] and fruit flies[42] when they encounter and cross gaps, for flight initiation of locusts[43], and the vision-induced landing response in flies[44]. Self-learning (operant conditioning), an important form of motor learning, depends on the activity of protein kinase C (PKC) in many animals and behaviors, including biting in Aplysia, song-learning in birds, procedural learning in mice and avoiding behaviors in flying fruit flies[45]. If flight in fruit flies is deprived within the first 3 days after hatching, the animals employ more corrective steering as an adult when flying towards visual objects[46]. Untrained, naïve fruit flies also reduce their maximum forward speed compared to controls and also loose their ability to precisely compensate their flight course for visual perturbations in the environment when flying freely under optomotor conditions. The loss in turning precision in naïve fruit flies, however, is not due to an impairment in power generation of A-IFM because maximum flight muscle force seems to be widely unchanged compared to controls. The latter finding runs counter to the idea that a loss in control precision is due to a loss of exercise, supporting the idea of synaptic plasticity for motor learning[46].
In conclusion, studies on neural precision of muscle activation in flies and thus on the question how graded visual signaling from the compound eyes is fused with spiking proprioceptive feedback from halteres and wings tackle not only principles of neural coding and timing. They address fundamental problems of sensory integration processes in fast-frequent locomotor systems. Sensory integration at the level of single motoneurons circumvents unwanted temporal delays in spike transmission, unavoidably occurring by the employment of more complex neural circuitries residing in the thoracic ganglia and CNS. The neural architecture for locomotor control in flies provides a system that does not only change muscle spike frequency but also dynamically controls the preferred spike phase from stroke-to-stroke and, consequently, the instantaneous work-phase gain of flight muscles. Interestingly, although gap junctions transmit excitatory feedback from halteres and wings during wing flapping, the two sensory signals cause opposing effects on wing motion control[47]. This finding implies that effective flight control in insects is due to a complex interplay between muscle-specific, nonlinear power generation and precise neural timing cues. The overall impact of these findings goes beyond insect flight since temporal delays within the nervous system also impedes locomotion in vertebrates. It is thus likely that the common neural principle for muscle control in the flight apparatus of flies is similar to the function of the local circuits for locomotor control found in the spinal chord of vertebrates[27].
References
- Willigenburg NW, Kingma I, Hoozemans MJ, et al. Precision control of trunk movement in low back pain patients. Hum Movement Sci. 2013; 32(1): 228-239.
- Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008; 211: 1792-1804.
- Faisal AA, Selen LPJ, Wolpert DM. Noise in the nervous system. Nature. 2008; 9: 292-303.
- Roth E, Sponberg S, Cowan NJ. A comparative approach to closed-loop computation. Curr Opin Neurobiol. 2013; 25: 54-62.
- Takei T, Seki K. Spinal premotor interneurons mediate dynamic and static motor commands for precision grip in monkeys. J Neurosci. 2013; 33(20): 8850-8860.
- Johansson R, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res. 1988; 71(1): 59-71.
- Koenraadt KLM, Roelofsen EG, Duysens J, et al. Cortical control of normal gait and precision stepping: An fNIRS study. NeuroImage. 2013; 85(1): 415-422.
- Fuglevand AJ. Mechanical properties and neural control of human hand motor units. J Physiol. 2011; 589(23): 5595-5602.
- Thelen DG, Anderson FC. Using computed muscle control to generate forward dynamic simulations of human walking from experimental data. J Biomech. 2006; 39(6): 1107-1115.
- Bradacs H, Kral K. Innervation of an insect asynchronous flight muscle as seen with scanning electron microscopy. Z mikroskop anatom Forsch. 1990; 104(2): 287-297.
- Rheuben MB, Kammer AE. Structure and innervation of the third axillary muscle of Manduca relative to its role in turning flight. J Exp Biol. 1987; 131(1): 373-402.
- Chakraborty S, Bartussek J, Fry SN, et al. Independently controlled wing stroke pattern in the fruit fly Drosophila melanogaster. PLoS one. 2015; 10(2): 1-29.
- Ellington CP. The aerodynamics of hovering insect flight. IV. Aerodynamic mechanisms. Phil Trans Roy Soc Lond B. 1984; 305: 79-113.
- Ristroph L, et al. Dynamics control and stabilization of turning flight in fruit flies. In: Natural locomotion in fluids and on surfaces. Springer New York. 2012; 83-99.
- Cheng B, Fry SN, Huang Q, et al. Aerodynamic damping during rapid flight maneuvers in the fruit fly Drosophila. J Exp Biol. 2010; 213(4): 602-612.
- Hesselberg T, Lehmann F-O. Turning behaviour depends on frictional damping in the fruit fly Drosophila. J Exp Biol 2007; 210: 4319-4334.
- Ramamurti R, Sandberg WC. A computational investigation of the three-dimensional unsteady aerodynamics of Drosophila hovering and maneuvering. J Exp Biol. 2007; 210: 881-896.
- Fry SN, Sayaman R, Dickinson MH. The aerodynamics of free-flight maneuvers in Drosophila. Science. 2003; 300: 495-498.
- Dickinson MH, Lehmann F-O, Götz KG. The active control of wing rotation by Drosophila. J Exp Biol. 1993;182: 173-189.
- Lehmann F-O, Götz KG. Activation phase ensures kinematic efficacy in flight-steering muscles of Drosophila melanogaster. J Comp Physiol. 1996; 179: 311-322.
- Tu MS, Dickinson MH. The control of wing kinematics by two steering muscles of the blowfly (Calliphora vicina). J Comp Physiol A. 1996; 178: 813-830.
- Sponberg S, Daniel TL. Abdication power for control: a precision timing strategy to modulate function of flight power muscles. Proc Roy Soc Lond B. 2012; 279: 3958-3966.
- Balint CN, Dickinson MH. The correlation between wing kinematics and steering muscle activity in the blowfly Calliphora vicina. J Exp Biol. 2001; 204: 4213-4226.
- Egelhaaf M. Visual afferences to flight steering muscles controlling optomotor responses of the fly. J Comp Physiol A. 1989; 165: 719-730.
- Springthorpe D, Fernándes M, Hedrick T. Neuromuscular control of free-flight turns in the hawkmoth Manduca sexta. J Exp Biol. 2012; 215(10): 1766-1774.
- Heide G. Neural mechanisms of flight control in Diptera. In: BIONA-report 2 ed Nachtigall W. Fischer Stuttgart. 1983; 35-52.
- Büschges A, Scholz H, El Manira A. New moves in motor control. Curr Biol. 2011; 21(13): 513-524.
- Dickinson MH, Farley CT, Full RJ, et al. How animals move: an integrative view. Science. 2000; 288: 100-106.
- Nalbach G, Hengstenberg R. The halteres of the blowfly Calliphora II. Three-dimensional organization of compensatory reactions to real and simulated rotations. J Comp Physiol A. 1994; 174: 695-708.
- Fox JL, Fairhall AL, Daniel TL. Encoding properties of haltere neurons enable motion feature detection in a biological gyroscope. PNAS. 2010; 107(8): 3840-3845.
- Ristroph L, Bergou AJ, Ristroph G, et al. Discovering the flight autostabilizer of fruit flies by inducing aerial stumbles. PNAS. 2010; 107(11): 4820-4824.
- Lehmann F-O, Bartussek J. Neural control and precision of flight muscle activation in Drosophila. J Comp Physiol A. 2017; 203: 1-14.
- Strausfeld NJ, Gronenberg W. Descending neurons supplying the neck and flight motor of Diptera: organization and neuroanatomical relationships with visual pathways. J Comp Neurol. 1990; 302: 954-972.
- Gronenberg W, Strausfeld NJ. Descending neurons supplying the neck and flight motor of Diptera: physiological and anatomical characteristics. J Comp Neurol. 1990; 302: 973-991.
- Trimarchi JR, Murphey RK. The shaking-B2 mutation disrupts electrical synapses in a flight circuit in adult Drosophila. J Neurosci Methods. 1997; 17(12): 4700-4710.
- Chan WP, Dickinson MH. Position-specific central projections of mechanosensory neurons on the haltere of the blowfly Calliphora vicina. J Comp Neurol. 1996 ;369: 405-418.
- Fayyazuddin A, Dickinson MH. Haltere afferents provide direct electrotonic input to a steering motor neuron of the blowfly Calliphora. J Neurosci. 1996; 16: 5225-5232.
- Suver MP, Huda A, Iwasaki N, et al. An array of descending visual interneurons encoding self-motion in Drosophila. J Neurosci. 2016; 36(46): 11768-11780.
- Hamer KC, Schreiber E, Burger J. Breeding biology life histories and life history-environment interactions in seabirds. Biol mar birds. 2001; CRC Press,Boca Raton: 217-261.
- Yoda K, Kohno H, Naito Y. Development of flight performance in the brown booby. Proc Roy Soc Lond B. 2004; 271(Suppl 4): 240-242.
- Blaesing B, Cruse H. Stick insect locomotion in a complex environment: climbing over large gaps. J Exp Biol. 2004; 207(8): 1273-1286.
- Pick S, Strauss R. Goal-driven behavioral adaptations in gap-climbing Drosophila. Curr Biol. 2005; 15: 1-6.
- Wilson DM. The central nervous control of flight in a locust. J Exp Biol. 1961; 38(47): 471-490.
- Borst A. How do flies land? BioScience. 1990; 40: 292-299.
- Colomb J, Brembs B. PKC in motorneurons underlies self-learning a form of motor learning in Drosophila. Peer J. 2016; 4: e1971.
- Hesselberg T, Lehmann F-O. The role of experience in flight behaviour of Drosophila. J Exp Biol. 2009; 212: 3377-3386.
- Bartussek J, Lehmann F-O. Proprioceptive feedback determines visuomotor gain in Drosophila. Roy Soc Open Sci. 2016; 3: 150562.
