An evolutionary perspective on habenular asymmetry in humans
Miguel L. Concha1,2,3*, Patricio Ahumada-Galleguillos1,2
Abstract
The habenula (Hb) of vertebrates is a dorsal and bilateral diencephalic nuclear complex that works as an anatomical hub integrating cognitive, emotional and sensory networks to regulate mood, motivation and value-based decision-making, among other functions. Across vertebrates, the Hb organises into two conserved separate components (medial and lateral in mammals equivalent to dorsal and ventral in more basal vertebrate species), which are thought to subserve different functions based on a partial independence of their connectivity systems. As a complex, the Hb shows morphological, molecular and connectivity differences between the left and right sides in a wide range of vertebrate species, which in some cases extend to the functional and behavioural levels. Habenular asymmetries are particularly prominent in basal vertebrate species but become less evident in amniotes and particular mammals. In humans, recent evidence reveals that, under an overall symmetry morphology, the Hb shows lateral differences in volume, activation, metabolism and susceptibility to damage that suggest an asymmetric condition of this nuclear complex. Here, we review the evidence supporting this view and discuss the possible origin of this asymmetric trait in humans from an evolutionary developmental perspective.
The habenula as a key regulator of mood, motivation and decision-making
In the brain of vertebrates, the habenula (Hb) is a dorsal and bilateral diencephalic nuclear complex that works as an anatomical hub that integrates cognitive, emotional and sensory networks to regulate mood, motivation and value-based decision-making, among other processes. In mammals, the left and right sides of the Hb are formed by medial (Med-Hb) and lateral (Lat-Hb) components which are thought to subserve different functions based on a partial independence of their afferent and efferent connectivity systems (Fig. 1A)1,2. The Med-Hb primarily receives inputs from the supracommissural septum and projects to the interpeduncular nucleus (IPN) in the ventral midbrain (Fig. 1A, green)3. The Lat-Hb, on the other hand, has more widespread connectivity linking the basal ganglia and limbic forebrain with brainstem dopaminergic (substantia nigra pars compacta, SN; ventral tegmental area, VTA), serotonergic (raphe nuclei), histaminergic (hypothalamus) and gabaergic (rostro medial mesopontine tegmental nucleus, RMTg) centres (Fig. 1A, red)4,5. Despite the significant amount of experimental data supporting the view of partial independence between the Med-Hb and Lat-Hb, there is nevertheless some degree of overlapping of afferent projections (i.e. descendent afferents from the accumbens and diagonal band nuclei, and ascendant afferents from the VTA and locus coeruleus)2,6 and interconnectivity7 between the two Hb domains.
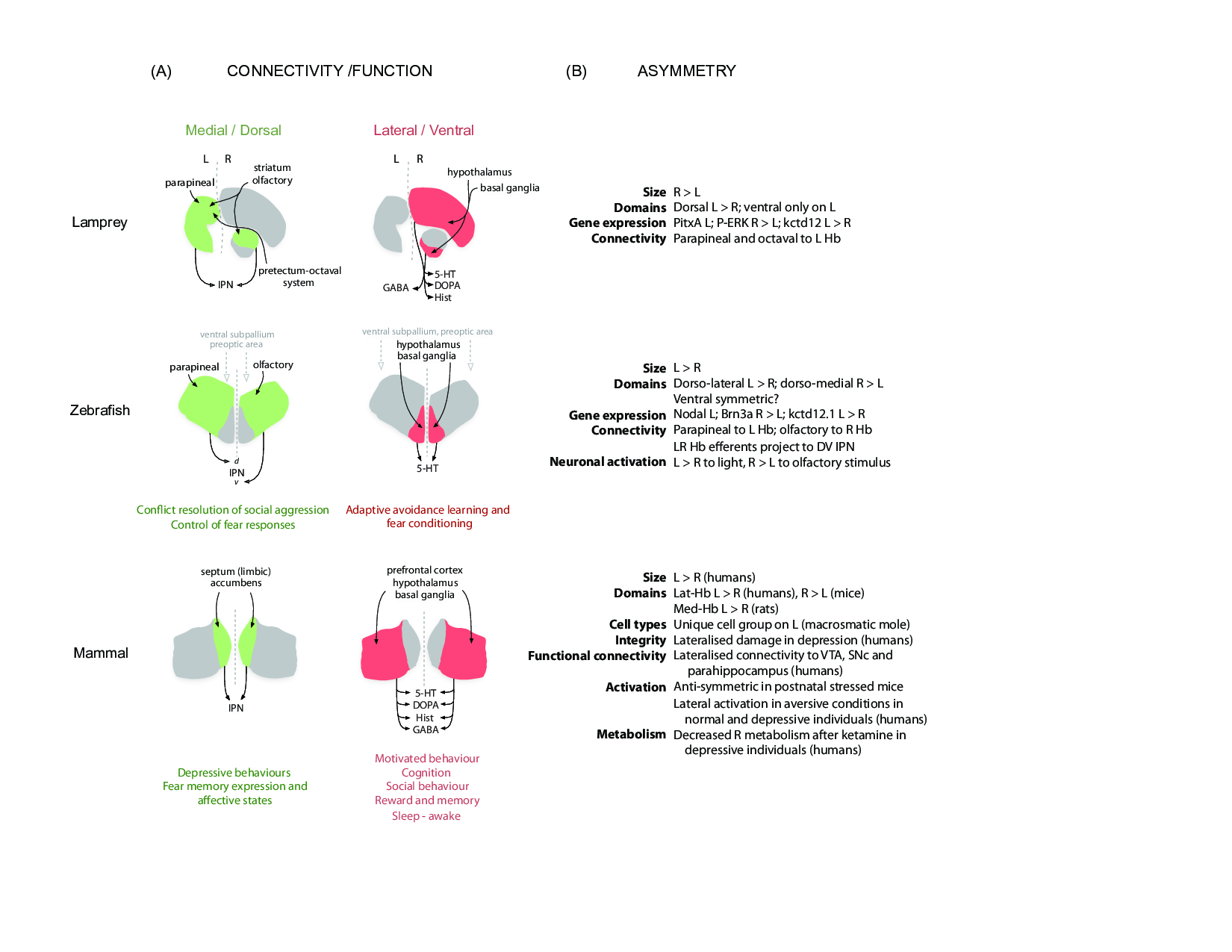
Figure 1: A comparative view of habenular connectivity, function and asymmetry in vertebrates.
(A) Organisation of connectivity and proposed function of the two main habenular pathways in vertebrates. Gene expression, neurochemistry and connectivity data from a basal vertebrate (lamprey), a modern teleost (zebrafish) and mammals (rodents and humans) support the existence of two partially independent habenular circuits. The medial habenular pathway of mammals is homologous to the dorsal habenular pathway of lampreys and zebrafish (left, green). On the other hand, the lateral habenular pathway of mammals is equivalent to the ventral habenular pathway of lampreys and zebrafish (right, red). The proposed motor-limbic and cognitive functions associated with each habenular domain are given below the schematics. (B) Left-right asymmetries of the Hb described at structural and functional levels in the lamprey, zebrafish and mammals (rodents and humans). Abbreviations: 5-HT (serotonergic), d (dorsal), DOPA (dopaminergic), GABA (gabaergic), Hist (histaminergic), IPN (interpeduncular nucleus), L (left), R (right), v (ventral). Details and references are given in the text.
Given the efferent connectivity pattern and the connection of its afferent septal nuclei with specific sectors of the hippocampus and subiculum, the mammalian Med-Hb has been involved in learning associated with fear responses and mood and to nicotine addiction, among other functions3. In turn, studies in rats and monkeys have shown that projections from the Lat-Hb to the VTA and SN provide reward prediction error and value related signals to dopaminergic systems that are fundamental to learning8,9. Studies in humans support this view and reveal Hb activation and increased functional connectivity between the Hb, VTA and SN in the context of reward predictor errors and negative motivation values associated with punishment10-13. In addition to learning, specific projections from the Lat-Hb to the serotonergic raphe nuclei provide long-term coding signals that appear to influence mood6,14. Consistently, structural and functional disruption of the Lat-Hb in humans has been linked to the origin of depression15-22 and substance abuse (cocaine and alcohol)23,24. Furthermore, deep brain stimulation of the Lat-Hb has proven successful to alleviate depressive symptoms in patients with treatment-resistant major depression25,26. Together, these findings reveal that as a complex, the Hb is an anatomical hub integrating cognitive, emotional and sensory networks and that in this context participates in the modulation of a series of motivated behaviours, including mood, learning and value-based decision-making.
Evolution of habenular circuits in vertebrates
Across vertebrates, the structural organisation of the Hb into two separate components seems to be an ancestral character, which is also closely related to the conservation of two segregated systems of habenular connectivity27-29. Particularly, the primary outputs to the IPN and to dopaminergic/serotonergic systems that characterise the medial and lateral domains of the mammalian Hb, respectively,2 are also present in the dorsal and ventral habenular domains of more basal vertebrate species such as lampreys and zebrafish (Fig. 1A)28-31. However, such conservation of the output circuit design differs from the evolutionary shift of inputs from sensorial (e.g. olfactory, parapineal, octaval system) to limbic, which is seen in the medial/dorsal habenular domain from basal vertebrates to mammals5,29,32-34. This observation suggests a change in the contextual information that is used by different species to activate habenular circuits. Also, in the evolution towards amniotes and specifically in mammals we observe a striking diversification of input sources to the Lat-Hb1,2, including the appearance of reciprocal projections from neuromodulatory monoaminergic habenular targets1,35. Interestingly, the relative size of the Lat-Hb is also significantly increased in humans compared with rats, such that it represents ∼95% of the total Hb volume36,37. Thus, there appears to be a growing diversification of afferent sources to the lateral/ventral habenular domain during evolution, in particular in mammals, which suggests an increasingly complex modulation of this habenular circuit. Although detailed studies of habenular connectivity in other vertebrate species are still needed, this idea finds support in the extensive range of functional/behavioural domains (i.e. sensorial, motivational and cognitive) in which the Lat-Hb has been involved in mammals and especially in humans5,9.
Asymmetry: a conserved but variable trait in the habenula of vertebrates
A noticeable and conserved feature of the bilateral Hb is the presence of morphological and molecular differences between the left and right sides (Fig. 1B)27. Although studies on habenular asymmetry have comprised a wide range of vertebrate species, they have mostly referred to the Hb as a complex and little information is available on the contribution of the medial/dorsal and lateral/ventral components to habenular asymmetry across species. As a complex, habenular asymmetry is morphologically conspicuous in agnathans and most cartilaginous and bony fishes27 and can involve both medial and lateral domains (e.g. lampreys)29 or be confined to a single domain (e.g. medial/dorsal Hb zebrafish)30. In vertebrate species other than fishes, and in particular in amniotes such as birds and mammals, the morphological asymmetries of the Hb are much less pronounced with only a few exceptions (e.g. lizards)27. In mammals, the presence of small lateral differences in habenular volume has been described in the Med-Hb of rats38 and the Lat-Hb of mice39, while a unique small group of cells was described on the left Hb of the macrosmatic mole40. In primates and in particular in humans, the study of habenular asymmetry is still in its infancy due to the small size and internal position of this nuclear complex within the brain. In spite of this, a recent post-mortem volumetric analysis revealed that the human Hb is significantly larger on the left compared to the right in both genders, a condition that results from an enlargement of the left Lat-Hb37. Consistent with these findings, a volumetric study using high-resolution magnetic resonance imaging (MRI) showed a tendency of increased left habenular volume in healthy subjects and individuals with various neuropsychiatric conditions, although these differences were statistically non-significant possibly due to the resolution limitations of this imaging technique17.
Asymmetry of the Hb does not restrict to the morphological and molecular domains but has also been observed at a functional level, suggesting that the Hb is involved in brain lateralisation. In zebrafish, for example, specific subsets of neurones on the dorsal Hb show differential calcium activation on the left and right sides in response to visual and olfactory stimuli, respectively41. Importantly, these functional asymmetries match the structural asymmetries in habenular morphology, gene expression and connectivity30,41, which have also been associated with visual-guided lateralised behaviours42. In mammals, recent studies reveal that the left and right Hb become differentially activated under specific conditions, but the anatomical substrate underlying habenular asymmetric activation is still unknown. In mice, the Hb becomes asymmetrically activated in postnatal mice under stress as revealed by the expression of early response genes in a transgenic background43. In humans, studies of high-resolution functional MRI have shown asymmetric blood oxygen level-dependent responses to aversive relative to neutral events13 and to negative conditioned stimulus values12. Furthermore, a recent report based on high-resolution cardiac-gated resting state imaging revealed that the right and left Hb have distinct correlates of functional connectivity with the VTA, SN and the parahippocampus44. In turn, studies in depressive patients show a significant decrease in activation of the left Hb during the prediction or experience of monetary penalty45, a decreased metabolism of the right Hb after ketamine treatment18, and a reduction of volume, neuronal cell number and cell area in histological postmortem samples16. Together, these findings suggest that the human Hb has a previously unrecognised asymmetric condition that comprises both structural and functional levels, which might be relevant in contexts of health and disease. In summary, morphological, molecular and connectional observations across vertebrates reveal the presence of notorious asymmetries in more basal species, which in some cases is linked to the asymmetric response of the Hb to sensorial stimuli and lateralised behaviours. In contrast, in the context of subtle morphological asymmetries, the Hb of mammals and particularly humans often shows asymmetries at a functional level but the anatomical substrate underlying this condition remains unknown.
Coding asymmetries on habenular circuits
From a functional viewpoint, a relevant question is how the different types of asymmetries are coded into circuits. Asymmetric circuits involving paired neuronal structures such as the Hb can in principle use two main designs: exhibit left-right differences in the extent of similar types of neurones/connectivity present on left and right sides (class-I), or show unique neurones or connectivity patterns on only one side of the brain (class-II)46. The Hb appears to shows both types of circuit designs in vertebrates. For example, the left and right Hb of a modern teleost such as zebrafish exhibit different proportions of similar neurones based on molecular markers and connectivity30. The presence of volumetric differences between the left and right sides of the Hb in humans37 and many other vertebrate species27 could also be indicative of a class-I asymmetry. On the other hand, the Hb of lampreys, frogs and lizards show unilateral cellular domains, i.e. the ventral Hb of lampreys is only on the right side29 while the laterodorsal and dorsomedial Hb domains of frogs47 and lizards48, respectively, are present only on the left27. On the other hand, a group of neurones of the olfactory bulb project only to the right Hb in zebrafish33,34 while parapineal neurones reach only the left Hb in lampreys, zebrafish and lizards32,48,49. Noteworthy, the observation of sided activation of the human Hb in certain functional tasks, the unilateral metabolic response to drugs and lateral damage in certain neuropsychiatric conditions (see above) suggest the presence of class-II asymmetric circuits in the human Hb.
In the context of the different asymmetric habenular designs, some elaborate patterns of connectivity have emerged. A striking example is provided by zebrafish, where the spatial segregation of left-right habenular outputs along the dorsoventral axis of the IPN define parallel tunnels of habenular information directed to the griseum centrale and raphe nuclei, respectively30,50. Significantly, habenular asymmetry and its coding into segregated efferent connectivity tunnels have been associated in this species with the asymmetric processing of visual and olfactory stimuli41,51, lateralised behaviours42, and the behavioural responses to fear and anxiety50,52. Whether similar asymmetric patterns of functional connectivity and behavioural correlates are associated with the Hb of other vertebrate species including humans is yet to be determined.
Insights into the origin and impact of habenular asymmetry in humans
An important lesson from the comparative studies described here is the existence of multiple types of habenular asymmetries in different species affecting various organisational levels, from gene expression through morphology and connectivity to function. In this context, it does not appear sensible to provide a single explanation for the origin of habenular asymmetry in humans. On a developmental ground, studies in fishes have suggested that the Hb shows independent developmental programs on the left and right sides that can be modulated unilaterally by genetic and cellular inputs to generate asymmetry during ontogeny53,54. For example, the left-sided activation of the transforming growth factor-ß Nodal in the embryonic brain controls for development of morphological, molecular and connectional asymmetries in lampreys, catshark and zebrafish53,55,56. Nodal signalling is a conserved pathway that regulates the development of body, visceral and brain asymmetries across metazoans in a context-dependent manner54,57,58, and in vertebrates, it has been associated with both enlarged and reduced habenular domains on the side of expression53,55,56. In addition to direct genetic inputs, the influence of an asymmetrically positioned cellular group, the parapineal organ (PpO), also plays a fundamental role in the elaboration of habenular asymmetries in zebrafish32,59,60. Notably, genetic (Nodal) and cellular (PpO) influences upon the developing Hb both seem to converge in the regulation of neurogenesis56,61. It is thus plausible that an asymmetric regulation of neurogenesis (via Nodal and other developmental inputs) is a conserved step in the origin of habenular asymmetry across vertebrates including humans. This idea is consistent with the reduction or evolutionary loss of gross morphological asymmetries observed in vertebrate groups other than fishes including mammals27, which follows the apparent loss of Nodal expression in the brain54. The mechanisms controlling habenular asymmetry in the absence of asymmetric Nodal signalling possibly exploit the sensitivity of the prospective Hb to spatial and temporal changes in genetic and cellular developmental inputs, as suggested by zebrafish studies32,60-63. These mechanisms could in principle generate structural and functional asymmetries in mammals beneath an apparent overall symmetric morphology of the Hb. The existence of a unique small group of cells on the left Hb of the macrosmatic mole40, of lateral differences in volume described in the Hb of rodents and humans37-39, of asymmetric functional connectivity in humans44, and asymmetric activation of the rodent and human Hb in different contexts of health and disease12,13,43,45 is consistent with this idea and supports the importance of considering other less evident types of habenular asymmetries in these species.
Functional studies involving areas such as the prefrontal cortex, amygdala, cerebellum and mesencephalon (i.e. optic tectum or colliculi) have shown that lateralisation is a key feature of the human brain64. In this context, demonstrating the existence of asymmetric circuit designs involving the human Hb is relevant to first understand the possible functional role of habenular asymmetry and then link this condition to a potential therapeutic use in the treatment of affective disorders. In contrast to sensorial asymmetries that in many cases associate with lateralised environmental stimuli, the mammalian Hb primarily integrates cognitive with sensorial and motivational information to generate an activation state that is contextually dependent, and thus the role of asymmetry in this process is less intuitive. The partial independence of left and right habenular circuits provides a substrate that could promote and perhaps explain the presence of sided activation and lateral susceptibility of the Hb to damage seen in humans. Should this is the case, it will become essential to distinguish the functions that the left and right Hb do in common from those that are unique to each of them. Future research will have to address these and other emerging questions and ascertain the presence and types of left-right asymmetric circuits in the human Hb and their correlates to habenular and brain function. To this aim, special efforts must be placed to improve the resolution of in vivo functional imaging and segmentation techniques, and to develop novel experimental designs that resolve left from right in the human Hb.
Acknowledgements
We thank Karina Palma for drawing the panels of the figure, and Iskra Signore for critical reading of the manuscript. This work was supported by the National Commission for Scientific and Technological Research (FONDECYT 1020902 and 3160486 to M.L.C and P.A.G., respectively; Ring Initiative PIA ACT1402 to M.L.C; Fondap 15150012 to M.L.C.), and the Millennium Science Initiative (P09-015-F to M.L.C).
References
- Sutherland RJ. The dorsal diencephalic conduction system a review of the anatomy and functions of the habenular complex. Neuroscience Biobehavioural Review. 1982; 6(1): 1-13.
- Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009; 364(1519): 1005-1020.
- Viswanath H, Carter AQ, Baldwin PR, et al. The medial habenula still neglected. Front Hum Neurosci. 2013; 7: 931.
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007; 31(5): 658-672.
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010; 11(7): 503-513.
- Boulos LJ, Darcq E, Kieffer BL. Translating the Habenula-From Rodents to Humans. Biol Psychiatry. 2016; http://dx.doi.org/10.1016/j.biopsych.2016.06.003.
- Kim U, Chang SY. Dendritic morphology local circuitry and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. Journal of Comparative Neurology. 2005; 483(2): 236-250.
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007; 447(7148): 1111-1115.
- Namboodiri VM, Rodriguez-Romaguera J, Stuber GD. The habenula. Curr Biol. 2016; 26(19): R873-R877.
- Salas R, Baldwin P, de Biasi M, et al. BOLD Responses to Negative Reward Prediction Errors in Human Habenula. Front Hum Neurosci. 2010; 4: 36.
- Ide JS, Li CS. Error-related functional connectivity of the habenula in humans. Front Hum Neurosci. 2011; 5: 25.
- Lawson RP, Seymour B, Loh E, et al. The habenula encodes negative motivational value associated with primary punishment in humans. Proc Natl Acad Sci U S A. 2014; 111(32): 11858-11863.
- Hennigan K, D'Ardenne K, McClure SM. Distinct midbrain and habenula pathways are involved in processing aversive events in humans. J Neurosci. 2015; 35(1): 198-208.
- Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17(9): 1146-1152.
- Morris JS, Smith KA, Cowen PJ, et al. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999; 10(2): 163-172.
- Ranft K, Dobrowolny H, Krell D, et al. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med. 2010; 40(4): 557-567.
- Savitz JB, Nugent AC, Bogers W, et al. Habenula volume in bipolar disorder and major depressive disorder a high-resolution magnetic resonance imaging study. Biol Psychiatry. 2011; 69(4): 336-343.
- Carlson PJ, Diazgranados N, Nugent AC, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression a preliminary positron emission tomography study. Biol Psychiatry. 2013; 73(12): 1213-1221.
- Carceller-Sindreu M, de Diego-Adelino J, Serra-Blasco M, et al. Volumetric MRI study of the habenula in first episode recurrent and chronic major depression. Eur Neuropsychopharmacol. 2015; 25(11): 2015-2021.
- Johnston BA, Steele JD, Tolomeo S, et al. Structural MRI-Based Predictions in Patients with Treatment-Refractory Depression (TRD). PLoS One. 2015; 10(7): e0132958.
- Ely BA, Xu J, Goodman WK, et al. Resting-state functional connectivity of the human habenula in healthy individuals: Associations with subclinical depression. Hum Brain Mapp. 2016; 37(7): 2369-2384.
- Schmidt FM, Schindler S, Adamidis M, et al. Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. Eur Arch Psychiatry Clin Neurosci. 2016; doi: 10.1007/s00406-016-0675-8
- Deserno L, Beck A, Huys QJ, et al. Chronic alcohol intake abolishes the relationship between dopamine synthesis capacity and learning signals in the ventral striatum. Eur J Neurosci. 2015; 41(4): 477-486.
- Parvaz MA, Konova AB, Proudfit GH, et al. Impaired neural response to negative prediction errors in cocaine addiction. J Neurosci. 2015; 35(5): 1872-1879.
- Sartorius A, Kiening KL, Kirsch P, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010; 67(2): e9-e11.
- Kiening K, Sartorius A. A new translational target for deep brain stimulation to treat depression. EMBO Mol Med. 2013; 5(8):1151-1153.
- Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J Anat. 2001; 199(Pt 1-2): 63-84.
- Amo R, Aizawa H, Takahoko M, et al. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci. 2010; 30(4): 1566-1574.
- Stephenson-Jones M, Floros O, Robertson B, et al. Evolutionary conservation of the habenular nuclei and their circuitry controlling the dopamine and 5-hydroxytryptophan (5-HT) systems. Proc Natl Acad Sci U S A. 2012;109(3): E164-173.
- Aizawa H, Bianco IH, Hamaoka T, et al. Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr Biol. 2005;15(3): 238-243.
- Turner KJ, Hawkins TA, Yanez J, et al. Afferent Connectivity of the Zebrafish Habenulae. Front Neural Circuits. 2016;10:30.
- Concha ML, Russell C, Regan JC, et al. Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron. 2003;39(3):423-438.
- Hendricks M, Jesuthasan S. Asymmetric innervation of the habenula in zebrafish. J Comp Neurol. 2007;502(4):611-619.
- Miyasaka N, Morimoto K, Tsubokawa T, et al. From the olfactory bulb to higher brain centers: genetic visualization of secondary olfactory pathways in zebrafish. J Neurosci. 2009; 29(15): 4756-4767.
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187(1):19-47.
- Diaz E, Bravo D, Rojas X,et al. Morphologic and immunohistochemical organization of the human habenular complex. J Comp Neurol. 2011; 519(18): 3727-3747.
- Ahumada-Galleguillos P, Lemus CG, Diaz E, et al. Directional asymmetry in the volume of the human habenula. Brain Struct Funct. 2016; doi: 10.1007/s00429-016-1231-z
- Wree A, Zilles K, Schleicher A. Growth of fresh volumes and spontaneous cell death in the nuclei habenulae of albino rats during ontogenesis. Anatatomy and Embryology. 1981; 161(4): 419-431.
- Zilles K, Schleicher A, Wingert F. [Quantitative growth analysis of limbic nuclei areas fresh volume in diencephalon and mesencephalon of an albino mouse ontogenic series. III. Nucleus interpe-uncularis]. Journal fur Hirnforschung. 1976; 17(1): 21-29.
- Kemali M. Morphological asymmetry of the habenulae of a macrosmatic mammal, the mole. Zeitschrift fur Mikroskopish Anatatomische Forschung. 1984; 98(6): 951-954.
- Dreosti E, Vendrell Llopis N, Carl M, et al. Left-right asymmetry is required for the habenulae to respond to both visual and olfactory stimuli. Curr Biol. 2014; 24(4): 440-445.
- Barth KA, Miklosi A, Watkins J,et al. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr Biol. 2005; 15(9): 844-850.
- Ichijo H, Hamada M, Takahashi S, et al. Lateralization, maturation, and anteroposterior topography in the lateral habenula revealed by ZIF268/EGR1 immunoreactivity and labeling history of neuronal activity. Neurosci Res. 2015; 95: 27-37.
- Hetu S, Luo Y, Saez I, et al. Asymmetry in functional connectivity of the human habenula revealed by high-resolution cardiac-gated resting state imaging. Hum Brain Mapp. 2016; 37(7): 2602-2615.
- Furman DJ, Gotlib IH. Habenula responses to potential and actual loss in major depression preliminary evidence for lateralized dysfunction. Soc Cogn Affect Neurosci. 2016; 11(5): 843-851.
- Concha ML, Bianco IH, Wilson SW. Encoding asymmetry within neural circuits. Nat Rev Neurosci. 2012; 13(12): 832-843.
- Braitenberg V, Kemali M. Exceptions to bilateral symmetry in the epithalamus of lower vertebrates. Journal of Comparative Neurology. 1970; 138(2): 137-146.
- Engbretson GA, Reiner A, Brecha N. Habenular asymmetry and the central connections of the parietal eye of the lizard. Journal of Comparative Neurology. 1981;198(1):155-165.
- Yañez J, Pombal MA, Anadon R. Afferent and efferent connections of the parapineal organ in lampreys: a tract tracing and immunocytochemical study. Journal of Comparative Neurology. 1999; 403(2): 171-189.
- Agetsuma M, Aizawa H, Aoki T, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. 2010; 13(11): 1354-1356.
- Krishnan S, Mathuru AS, Kibat C, et al. The right dorsal habenula limits attraction to an odor in zebrafish. Curr Biol. 2014; 24(11): 1167-1175.
- Facchin L, Duboue ER, Halpern ME. Disruption of Epithalamic Left-Right Asymmetry Increases Anxiety in Zebrafish. J Neurosci. 2015; 35(48): 15847-15859.
- Lagadec R, Laguerre L, Menuet A, et al. The ancestral role of nodal signalling in breaking L/R symmetry in the vertebrate forebrain. Nat Commun. 2015; 6: 6686.
- Signore IA, Palma K, Concha ML. Nodal signalling and asymmetry of the nervous system. Philos Trans R Soc Lond B Biol Sci. 2016; doi: 10.1098/rstb.2015.0401
- Concha ML, Burdine RD, Russell C, et al. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000; 28(2): 399-409.
- Roussigne M, Bianco IH, Wilson SW, et al. Nodal signalling imposes left-right asymmetry upon neurogenesis in the habenular nuclei. Development. 2009;136(9):1549-1557.
- Grande C, Martin-Duran JM, Kenny NJ, et al. Evolution divergence and loss of the Nodal signalling pathway new data and a synthesis across the Bilateria. Int J Dev Biol. 2014; 58(6-8): 521-532.
- Shiratori H, Hamada H. TGFbeta signaling in establishing left-right asymmetry. Semin Cell Dev Biol. 2014; 32: 80-84.
- Gamse JT, Thisse C, Thisse B, et al. The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development. 2003;130(6):1059-1068.
- Concha ML, Signore IA, Colombo A. Mechanisms of directional asymmetry in the zebrafish epithalamus. Semin Cell Dev Biol. 2009; 20(4): 498-509.
- Aizawa H, Goto M, Sato T, et al. Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev Cell. 2007;12(1): 87-98.
- Husken U, Stickney HL, Gestri G, et al. Tcf7l2 is required for left-right asymmetric differentiation of habenular neurons. Curr Biol. 2014; 24(19): 2217-2227.
- Signore IA, Concha ML. Heterochrony and Morphological Variation of Epithalamic Asymmetry. The Journal of Experimental Zoology Part B. 2016;doi: 10.1002/jez.b.22698.
- Rogers LJ, Vallortigara G, Andrew RJ. Divided Brains. Cambridge: Cambridge University Press; 2013.
